10-K: Annual report pursuant to Section 13 and 15(d)
Published on February 28, 2022
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM
(Mark One)
ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
|
For the fiscal year ended |
|
TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
|
For the transition period from to . |
Commission File Number:

(Exact Name of Registrant as Specified in its Charter)
|
(State or other jurisdiction of incorporation or organization) |
(I.R.S. Employer Identification No.) |
(Address of principal executive offices, including zip code)
(
(Registrant’s telephone number, including area code)
Securities registered pursuant to Section 12(b) of the Act:
Title of Each Class |
Trading Symbol(s) |
Name of Each Exchange on Which Registered |
||
The |
Securities registered pursuant to Section 12(g) of the Act: Warrants to purchase Common Stock
Indicate by check mark if the registrant is a well-known seasoned issuer, as defined in Rule 405 of the Securities Act.
Indicate by check mark if the registrant is not required to file reports pursuant to Section 13 or Section 15(d) of the Act. Yes ◻
Indicate by check mark whether the registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days.
Indicate by check mark whether the registrant has submitted electronically every Interactive Data File required to be submitted pursuant to Rule 405 of Regulation S-T (§ 232.405 of this chapter) during the preceding 12 months (or for such shorter period that the registrant was required to submit such files).
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, a smaller reporting company, or an emerging growth company. See the definitions of “large accelerated filer,” “accelerated filer,” “smaller reporting company,” and “emerging growth company” in Rule 12b-2 of the Exchange Act.
☒ |
Accelerated filer |
◻ |
|
|
|
|
|
Non-accelerated filer |
◻ |
Smaller reporting company |
|
|
|
|
|
|
|
Emerging growth company |
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act. ◻
Indicate by check mark whether the registrant has filed a report on and attestation to its management’s assessment of the effectiveness of its internal control over financial reporting under Section 404(b) of the Sarbanes-Oxley Act (15 U.S.C. 7262(b)) by the registered public accounting firm that prepared or issued its audit report.
Indicate by check mark whether the registrant is a shell company (as defined in Rule 12b-2 of the Exchange Act). Yes
The aggregate market value of common stock held by non-affiliates of the registrant as of June 30, 2021 was $
As of February 18, 2022, there were
DOCUMENTS INCORPORATED BY REFERENCE
Portions of the registrant’s proxy statement for the 2022 Annual Meeting of Stockholders are incorporated herein by reference in Part III of this Annual Report on Form 10-K where indicated. Such proxy statement will be filed with the U.S. Securities and Exchange Commission within 120 days of the registrant’s fiscal year ended December 31, 2021.
TABLE OF CONTENTS
2
FORWARD-LOOKING STATEMENTS
References to the “Company,” “Cryoport,” “we,” “us,” “our” and other similar words refer to Cryoport Inc. and its consolidated subsidiaries, unless the context suggests otherwise. This Annual Report on Form 10-K (this “Form 10-K”) contains certain forward-looking statements. These forward-looking statements involve a number of risks and uncertainties. These forward-looking statements can generally be identified as such because the context of the statement will include certain words, including but not limited to, “believes,” “may,” “will,” “expects,” “intends,” “estimates,” “anticipates,” “plans,” “seeks,” “continues,” “predicts,” “potential,” “likely,” or “opportunity,” and also contains predictions, estimates and other forward-looking statements within the meaning of Section 27A of the Securities Act of 1933, as amended (the “Securities Act”), and Section 21E of the Securities Exchange Act of 1934, as amended (the “Exchange Act”), and in reliance upon the safe harbor provisions of the Private Securities Litigation Reform Act of 1995. Such forward-looking statements are based on the current beliefs of the Company’s management, as well as assumptions made by and information currently available to the Company’s management. Readers of this Form 10-K should not put undue reliance on these forward-looking statements, which speak only as of the time this Form 10-K was filed with the Securities and Exchange Commission (the “SEC”). Reference is made in particular to forward-looking statements regarding our expectations about future business plans, new products or services, regulatory approvals, strategies, development timelines, prospective financial performance and opportunities, including potential acquisitions, expectations about future benefits of our acquisitions, including Cryogene, CRYOPDP and MVE Biological Solutions, our ability to successfully integrate those businesses and our plans related thereto; liquidity and capital resources; projected trends in the market in which we operate; anticipated impacts from the coronavirus strain COVID-19 (“COVID-19”) on us, including to our business operations, results of operations, cash flows, and financial position, and our future responses to the COVID-19 pandemic; our expectations about securing and maintaining strategic relationships with global couriers or large clinical research organizations; our future capital needs and ability to raise capital on favorable terms or at all; results of our research and development efforts; and approval of our patent applications. Forward-looking statements are inherently subject to risks and uncertainties, some of which cannot be predicted or quantified. The Company’s actual results may differ materially from the results projected in the forward-looking statements. Factors that might cause such a difference include, but are not limited to, those discussed in this Form 10-K, including the “Risk Factors” in “Part I, Item 1A — Risk Factors”, and in “Part II, Item 7 — Management’s Discussion and Analysis of Financial Condition and Results of Operations.”
Past financial or operating performance is not necessarily a reliable indicator of future performance, and you should not use our historical performance to anticipate results or future period trends. We can give no assurances that any of the events anticipated by the forward-looking statements will occur or, if any of them do, what impact they will have on our results of operations and financial condition. Except as required by law, we do not undertake to update any such forward-looking statements and expressly disclaim any duty to update the information contained in this Form 10-K.
3
PART I
Item 1. Business
Overview
Cryoport Inc. (“Cryoport,” “we,” “our,” or the “company”) is a global leader in serving the life sciences industry as a trusted provider of integrated temperature-controlled supply-chain solutions supporting the biopharma/pharma, animal health, and reproductive medicine markets. Our mission is to support life and health worldwide and we are continuously developing, implementing, and leveraging our supply chain platform, which is designed to deliver comprehensive, unparalleled, highly differentiated temperature-controlled logistics, packaging, storage, cryogenic systems, informatics, and related services for life science products, regenerative medicine, cellular therapies, and treatments that require unique, specialized cold chain management.
Spread across 15 countries and 33 locations worldwide, we serve more than 3,000 customers working in biopharmaceutical, animal husbandry and reproductive medicine companies, universities, research institutions and government agencies. Our platform of solutions together with our global team of more than 850 colleagues delivers a unique combination of innovative supply chain technologies and services through our industry-leading brands, Cryoport Systems, CryoStork®, MVE Biological Solutions, CRYOPDP, and CRYOGENE.
Cryoport’s advanced supply chain platform, comprised of comprehensive and technology-centric systems and solutions, are designed to support the global high-volume distribution of commercial biologic and cell-based products and therapies regulated by the United States Food and Drug Administration (FDA) and other international regulatory bodies for distribution in the Americas, EMEA (Europe, the Middle East, and Africa) and APAC (Asia-Pacific) regions. Cryoport’s solutions are also designed to support pre-clinical, clinical trials, Biologics License Applications (BLA), Investigational New Drug Applications (IND) and New Drug Applications (NDA) with the FDA, as well as global clinical trials initiated in other countries, where strict regulatory compliance and quality assurance is mandated. Over the last several years, we have grown to become a leader in supporting the clinical trials and commercial launches of cell and gene therapies globally. As of the end of the fiscal year of 2021, we supported 602 clinical trials and eight commercial therapies, including KYMRIAH by Novartis, YESCARTA and TECARTUS by Gilead/Kite, and BREYANZI and ABECMA by Bristol-Myers Squibb. A total of eleven (11) Cryoport-supported Biologic License Applications (BLAs) or Marketing Authorization Applications (MAAs) were filed in fiscal year 2021, based on internal information and forecasts from the Alliance for Regenerative Medicine, of which two (2) were filed during the fourth quarter of 2021. Looking forward, we anticipate up to nineteen (19) BLA and MAA submissions for Cryoport-supported products during 2022. Those therapies that receive commercial approval will provide opportunities to become significant revenue drivers for us in the future as the majority of them will require temperature-controlled storage, comprehensive temperature-controlled supply chain support and other services at commercial scale, and we expect that many will select us as their critical supply chain solution as a result of our work in connection with their respective clinical trials and track record in the market.
Cryoport’s advanced supply chain platform also supports the animal health market and the human reproductive market. The animal health market is mainly composed of supporting animal husbandry, as well as companion and recreation animal health. The human reproductive market which is largely composed of In-Vitro Fertilization (IVF) support for patients and clinics.
Our industry standard setting Chain of Compliance® solutions, which include vital analytics, such as ‘chain-of-condition’ and ‘chain-of-custody’ information in a single data stream, empower our clients’ continuous vigilance over their respective commodities. In addition, our Chain of Compliance® standard ensures full traceability of the equipment used and the processes employed during storage, fulfillment, and distribution, further supporting each client’s goal of minimizing risk and maximizing success of their respective new biologics or other products and therapies as they are introduced into the global markets.
As part of our services, our platform of technologies provides the ability for Cryoport personnel, and our clients, to monitor conditions of the internal physical environment, geographic location and other specified critical variables for each shipment or sample in near real time. In accordance with client requirements, information is recorded and archived for each shipment or sample for scientific, quality assurance and regulatory purposes in a secure cloud-based system that can be accessed by authorized personnel globally. This information provides an audit trail that can verify the in-shipment or sample condition in which the life sciences commodity, material, product, vaccine, or therapy was shipped and/or stored.
4
One of the most important features of our supply chain solutions platform is our suite of sophisticated, cloud-based, logistics management platforms, which are branded as the Cryoportal® Logistics Management Platform (the “Cryoportal®”), and CRYOPDP UnITy platform, both of which are supported by an integrated control tower. These platforms support the management of shipments through a single interface, which includes order entry, document preparation, customs documentation, courier management, near real-time shipment tracking and monitoring, issue resolution, and regulatory compliance requirements. In addition,they provide unique and incisive information dashboards and validation documentation for every shipment through data collected by the SmartPak ™ Condition Monitoring System(s) (the “SmartPak™”). The Cryoportal® can record and retain a fully documented history of all Cryoport Express® and ELITE™ Shippers, including chain-of-custody, chain-of-condition, chain-of-identity, and Chain of Compliance™ information for each shipment, which is used to ensure that the stability of shipped biologic commodities are maintained throughout the shipping cycle. At the client’s option, recorded information is archived, allowing the client to meet exacting requirements necessary for scientific work and/or proof of regulatory compliance during the logistics process. CRYOPDP’s UnITy transportation management platform contains various modules that include a tracking, order management, asset management, all of which report up to a main control tower to ensure full supply chain visibility and supporting analytics.
Cryoport’s MVE Biological Solutions systems are an important part of our global supply chain platform. MVE Biological Solutions is the leading global manufacturer of temperature-controlled storage and distribution equipment and systems. MVE has set the standard for the manufacture of cryogenic systems including vacuum insulated products and cryogenic freezer and shipper solutions used for storage and/or distribution of critical biological material for more than 50 years. MVE Biological Solutions’ equipment is used extensively throughout the life sciences industry and is the trusted solution within the regenerative medicine space for the storage and distribution of cell and gene therapies. Moreover, Cryoport Systems in conjunction with MVE Biological Solutions are developing a new platform of ultra-low temperature smart packaging for cell and gene therapy distribution through a new purpose built Cryoport ELITE™ product line. The first two products in the Cryoport ELITE™ line to launch will be the Cryosphere™, which is a first of its kind gravitationally stabilized cryogenic shipper aimed at supporting of cell therapies and a novel ELITE™-80°C shipper, which, is purpose built for the support of gene therapy distribution and upstream viral vector products. Both these products are being launched to complement our current Cryoport Express® and CRYOPDP Temperature Controlled Packaging Solutions.
Our advanced technologies and dedicated personnel allow us to continue to expand our services footprint with a growing suite of services, products and competencies supporting the life sciences industry, which currently include information technology, primary and secondary packaging, analytics, logistics distribution, laboratory relocation, biostorage services, embedded logistics support and validation services (e.g., for shipping lanes and packaging) and consulting services. A sample of our client facing, supply-chain solutions include the following service platforms:
| ● | Industry leading temperature-controlled packaging and data management at all temperatures via MVE, Cryoport Systems and CRYOPDP |
| ● | Logistics planning, management, and transportation of critical materials globally via Cryoport Systems and CRYOPDP |
| ● | cGMP storage and distribution (equipment and services) of life science materials via CRYOGENE, MVE and Cryoport Systems |
| ● | Kitting, labelling, drug return and return therapy destruction via Cryoport Systems |
| ● | Consulting services in support of custom primary and secondary packaging, as well as packaging validation and testing services via Cryoport Systems |
These service platforms have been designed to effectively support, for example, the following use cases:
| ● | Cell-based Autologous Immunotherapy (Personalized Medicine) Solutions, designed for therapies in which our Cryoport ELITE™, Cryoport Express®, and CRYOPDP Solutions serve as an enabling technology for the safe and efficient storage and transportation of leukapheresis or apheresis blood products as well as the manufactured autologous cellular-based immunotherapies. This is accomplished by providing a comprehensive logistics solution for the verified chain-of-condition, chain-of-custody, chain-of-identity, and Chain of Compliance® transport from, (a) the collection of the patient’s blood or cells at a point-of-care setting, to (b) a central processing facility where they are manufactured into a personalized medicine, to (c) the safe, cryogenically preserved delivery of these often irreplaceable cells to a point-of-care treatment facility for infusion into the patient. The Advanced Therapy Shippers™ and Cryoport ELITE™ Shippers are designed specifically for this market. If required, Cryoport Express® Shippers can also serve as a temporary freezer/repository supporting the efficient distribution of the personalized medicine to the patient when and where the medical provider needs it, without the expense and inconvenience of on-sight, cryopreservation storage freezers. |
5
| ● | Allogeneic Therapy Solutions, designed for allogeneic therapies in which our Cryoport ELITE™, Express®, and CRYOPDP Solutions serve as enabling technologies for the safe and efficient storage and transportation of healthy donor blood products as well as the manufactured allogeneic therapies by providing a comprehensive logistic solutions for the verified chain-of-condition, chain-of-custody, chain-of-identity, and Chain of Compliance® transport from, (a) the blood collection center, to (b) the manufacturing facility for the allogeneic therapy, to (c) a storage and fulfillment facility, or (d) to a point-of-care treatment facility for infusion into the patient. This is another market where the Cryoport Systems’ Advanced Therapy Shipper™ and Cryoport ELITE™ shippers will play a role. |
| ● | Gene Therapy Solutions, designed for gene therapies in which our Cryoport ELITE™ solution has been purpose designed to be a best-in-class platform for the safe and efficient transportation of gene therapy products at -80°C by providing comprehensive packaging, logistics, and storage solutions for the verified chain-of-condition, chain-of-custody, chain-of-identity, and Chain of Compliance® transport from, (a) the manufacturing facility for the gene therapy, to (b) a storage and fulfillment facility, or (c) to a point-of-care treatment facility for infusion into the patient. |
| ● | Direct-to-Patient Solutions, designed for therapies and/or programs that require distribution to a residential or community care setting. Our CRYOPDP unit has developed specific processes and services in support of Direct-to-Patient requirements that include regulatory, security, accessibility, good distribution practices (GDP), and confidentiality considerations. |
| ● | Consulting Services, provides our clients an opportunity to leverage our in-house talent and knowledge to design custom logistics plans, perform lane assessment, lane and carrier validation; design custom packaging and validation, permitting clinical trial logistics design; commercial launch planning; systems integration; and end user training. Additionally, our Consulting Services team has developed a “Packaging Center of Excellence” in support of the advanced therapies space. |
Cryoport’s mission is to enable the life sciences to save and improve lives around the world by providing certainty throughout the supply chain – one patient, one therapy, one product at a time. Our people, innovative solutions, and industry leading technologies have been designed to exceed current standards to deliver certainty and de-risk the process across the entire supply chain for the life sciences.
Competitive Advantages
With our first-to-market integrated platform of services, technology-driven supply-chain solutions and decades of experience serving the life sciences industry, we have established a substantial lead over potential competitors. Furthermore, we are not aware of any company that offers services comparable to Cryoport’s full platform of solutions, capabilities, or competencies. Working with our depth of knowledge in information technology, bioservices, packaging, temperature-controlled logistics, and cryogenic systems, our management, technical, business development and service support teams approach our growing markets with adaptability, innovation, and creative thinking.
The most common alternatives to Cryoport’s platform of solutions are “older technologies” and/or systems as well as cobbled together, non-integrated platforms. In fact, a portion of the biopharma market and much of the animal health market still uses liquid nitrogen in its hazard liquid form and/or dry ice with no ongoing validation processes for equipment or procedures. In the case of dry ice, the technology delivers temperatures of approximately -80℃ with standard deviations up to 14℃. Consequently, it provides an environment that allows cellular activity to continue and cells to degrade, impacting cell line performance and cell viability. On the other hand, liquid nitrogen, while effective in holding cryogenic temperatures, is bulky, heavy, expensive and requires special handling to avoid spillage and accommodate weight restrictions. Both dry ice and liquid nitrogen are classified “hazardous” by IATA and, therefore, are also classified as “dangerous goods,” requiring additional permits and fees. Cryoport’s cryogenic solutions on the other hand are classified as non-hazardous. Additionally, cobbled together systems with assets and technologies managed by multiple entities introduces gaps in the compliance platform of the offering which in turn creates inherent material risks during the storage, fulfillment, and/or transport processes.
6
Through our experience, we know that the logistics distribution process can have a large impact on product/commodity conditions. This is especially important for the high value and at times irreplaceable commodities that we transport, whether in support of a clinical trial or the commercial distribution of a product. We therefore go beyond traditional ISTA (International Safe Transit Association) packaging validation and have implemented Quality-by-Design processes that allow us to assess in-field events, the impact of logistics on the commodity being shipped, and the equipment being used for each individual shipment. With the acquisition of CRYOPDP, Cryoport now has more control and accountability around the logistics distribution which in turn provides better performance and risk management for our clients and their critical therapies.
We have been qualified as a trusted temperature-controlled solutions provider for hundreds of life sciences companies, institutions, and governments and, as of December 31, 2021, supported over 602 clinical trials in the regenerative medicine space. Cryoport and CRYOPDP have logged over 500,000 shipments to over 100 countries with hundreds of different types of life sciences materials in the last 12 months alone. Our experience and reputation, combined with decades of know-how and technology, provides us with significant competitive advantages. As an example, Novartis, Kite Pharmaceuticals Inc. (a subsidiary of Gilead Sciences), Bluebird Bio, Orchard Therapeutics, and Bristol Myers Squibb have all entrusted Cryoport to manage their respective global clinical shipments of gene and cell therapies trials and the commercial shipments of their respective therapies, KYMRIAH®, YESCARTA®, TECARTUS™, BREYANZI®, ABECMA®, LIBMELDY™, and ZYNTEGLO™. Cryoport is now supporting eight commercially approved products in the regenerative medicine space.
Cryoport Systems’ Cryoportal® logistics management platform (“Cryoportal”) is an important backbone technology that is integrated with our partners like FedEx, UPS, DHL, McKesson, Be-The-Match Biotherapies, Lonza, and others. The Cryoportal® handles order entry, keeps track of our global inventory, and provides algorithms for predictive analysis on every shipment while in transit, globally. Our customer service team monitors every shipment 24/7/365 while they are in transit and, by leveraging the Cryoportal, they have the unique ability to see issues that arise and take corrective measures up to and including intervention to save a shipment when needed.
Embedded within the Cryoportal® is the Chain of Compliance®, which is important for regulatory reasons and for risk mitigation. Using our Chain of Compliance®, every Cryoport Express® and ELITE™ shipper has its unique ID, every SmartPak® condition monitoring system has a unique ID, everything that is used more than once has a unique ID that is attached to it for its entire life. Thereby, Cryoport personnel can pull any shipper out of our inventory and tell the customer its entire history including every journey it has taken, for whom it was shipped, the contents shipped, the Cryoport shipper’s performance during transit, and the time of its return to a Cryoport Systems Global Logistics facility. It then continues to provide information on who performed the validated cleaning process, who recertified the unite and its components, and who recalibrated the SmartPak®, and who recertified it as being acceptable for its next use. All this traceability is stored in our Cryoportal® for our clients to access at any time. We are unaware of any other company that provides this solution.
In October 2020, we made two acquisitions: CRYOPDP and MVE. The CRYOPDP acquisition, in October 2020, added 25 facilities located in 14 countries to Cryoport bringing us to a total of 33 facilities in 15 countries. Our network of logistics centers provides redundancies for safety and certainty and enables us to be closer to our clients. It is our goal to have facilities within close proximity to global cell and gene manufacturing sites. With the CRYOPDP acquisition we instantly expanded our global network to cover most of the important locations. CRYOPDP and Cryoport Systems have already initiated the process of establishing jointly run facilities when appropriate to provide additional efficiencies and flexibility to our mutual client base. The first facilities that are jointly run include Osaka, Japan, Singapore, and Morris Plains, NJ.
Approximately eight years ago Cryoport Systems outsourced the manufacturing of the Cryoport Express® cryogenic dewars to the MVE, then a division of Chart Industries. At the time, Cryoport selected MVE because we felt that they were the best at cryogenic manufacturing, produced the highest quality products, and could scale to meet our volume requirements over time. By acquiring MVE, Cryoport became vertically integrated with the leading provider of cryogenic systems in the world and we secured one of our primary strategic inventory sources, which assures us of our ability to scale and further develop our Chain of Compliance® .
Today, Cryoport is the leading temperature-controlled supply chain solutions company serving the life sciences industry with a focus on the fast-paced cell and gene therapies market. In regenerative medicine, we support the major share of clinical trials; we support the most commercial therapies; we have a global logistics of network of 33 facilities, and we own the largest cryogenic systems manufacturing company for the life sciences in the world. We are forecasting a record number of regenerative therapies to file for approval this year and the cell and gene market is expected to grow rapidly for the next decade and beyond. We think Cryoport is well positioned to benefit from that growth -- today and for years to come.
7
Cryoport Products and Services
Cryoport is known as an industry leader in bringing best-in-class, comprehensive temperature-controlled supply chain solutions to the biopharma, reproductive medicine, and animal health markets. We have been successful in capturing significant market share through the development and implementation of a growing suite of supply-chain solutions with a relentless focus on effective risk management through market leading systems, processes, technology, and informatics systems coupled with excellent customer service in the management of our clients’ irreplaceable commodities. Our suite of market leading products and services include, but are not limited to the following:
Cryoport Express® Shippers - Cryoport Express® Shippers range from liquid nitrogen dry vapor shippers (-150℃) to our C3™ Shippers (2-8℃), which are powered by phase-change materials. The Cryoport Express® Shippers are precision-engineered assemblies that are reliable, cost-effective, and reusable or recyclable. Our liquid nitrogen dry vapor Cryoport Express® Shippers utilize an innovative application of ‘dry vapor’ liquid nitrogen technology and, most often, include a SmartPak™ Condition Monitoring System. Our Cryoport Express® Shippers are purpose built. Cryoport Express® Shippers meet International Air Transport Association (“IATA”) requirements for transport, including Class 6.2 infectious substances. Cryoport Express® Shippers are also International Safe Transit Association (“ISTA”) “Transit Tested” certified and carry the CE (“Conformité Européenne”) mark demonstrating conformance with European Union health, safety, and environmental protection standards.
Cryoport ELITE™ Shippers – Cryoport has designed a best-in-class family of -80°C shippers that have superior temperature management properties as well as incorporate next generation protection, handling, and data collection and management systems including our SmartPak™ Condition Monitoring System. The ELITE™ shipper line has been developed in conjunction with one of the leaders in the gene therapy space for clinical and commercial gene therapy distribution. The ELITE™ shipper platform will be launched in the first half of 2022.
Cryoport Cryosphere™ – The Cryosphere is a revolutionary gravitationally stabilized Cryoport Express® Shipper that is expected to be the most advanced cryogenic shipper to support the cell and gene therapy and other life sciences markets. The shipper is designed to passively stabilize the payload through its internal gravitational sphere, thereby keeping the payload in an upright orientation regardless of the external shipper orientation. This innovative technology further mitigates one of the key risks during storage, handling, and transport, which is maintaining constant cryogenic temperatures. In addition, the Cryosphere will have advanced shock and vibration absorption properties to further protect the payload and will be outfitted with Cryoport’s SmartPak™ state-of-the-art condition monitoring systems. It has also been designed to be ergonomically friendly, thus providing a better user experience than other products in the market. The Cryosphere™ is expected to be launched during the first half of 2022.
Cryoport Consulting Services – Cryoport Consulting Services functions in an expert advisory capacity to offer solutions to address risk factors present in temperature-controlled supply chain and logistics. To develop tailored scalable solutions, our cross-functional team collaborates with our clients to understand supply chain, logistics, time, shipper and packaging concerns. Consulting Services employs a structured approach to managing, executing, and developing risk mitigation plans. Our clients benefit from our quality driven processes and solutions delivered by our high integrity team leveraging industry-standard best practices and years of experience partnering with leading regenerative medicine companies from early clinical through post-commercialization. Service solutions range from comprehensive physical, thermal and shipping qualifications of shipping systems and/or packaging to developing user-friendly custom packaging solutions focused on the challenges unique to our Regenerative Medicine customers. Our Packaging Center of Excellence is expected to launch in 2022 to better serve our clients in Biopharmaceuticals, Animal Health and Reproductive Medicine by providing state-of-the-art customized packaging, testing, and qualification capabilities.
Cryoport Bioservices – With the upcoming launch of our new Cryoport Supply Chain Centers during the first half of 2022, Cryoport Systems will launch its Bioservices business. The new Cryoport Supply Chain Centers will offer a fully new and integrated approach designed to support cell and gene therapies including comprehensive controlled temperature storage, fulfilment, kit production, secondary packaging, labelling of therapeutic products and GMP raw materials storage along with world class logistics in both Houston, Texas and Morris Plains, New Jersey. In summary, these state-of-the-art facilities will combine our existing logistics processes and capabilities with our new, cutting edge Bioservices infrastructure – all under one roof, as Cryoport’s Global Supply Chain Center Network. Further expansion of the Cryoport Global Supply Chain Center Network is expected to include additional sites in the Americas, EMEA and APAC regions. The addition of these facilities and services provides for our clients’ increasing need for comprehensive and integrated solutions offerings and the expected growth in the global biostorage and bioservices markets, which are driven by the acceleration of clinical trials and the commercialization of regenerative medicine therapies on a global basis.
8
CRYOGENE - provides unparalleled solutions for the provision of pre-clinical temperature-controlled biological materials management services to the life science industry. These services include comprehensive specimen storage, processing, collection, and retrieval at our recently expanded CRYOGENE operations in Houston, TX, which is a cGMP-compliant operation.
CRYOPDP Temperature Controlled Logistics - CRYOPDP is a Specialist providing global and innovative temperature-controlled logistics solutions to the Clinical Research and Cell & Gene Therapy Communities. CRYOPDP operates with expertise an exhaustive range of temperature-controlled logistics services including Temperature controlled Packaging and Premium transport solutions from Cryogenic temperature (-196°C to 150°C) to Controlled Ambient (+15°C to +25°C).
CRYOPDP Direct to Patient Solutions - Direct-to-Patient is an innovative solution designed to meet the requirements of decentralized clinical trials, by focusing on the patient and customer-specific needs. This tailor-made solution allows the patients to access their drug therapies in the comfort of their own home, delivering significant benefits for the patient and to all the associated parties, by reducing drop-out rates due to lack of accessibility or time. Direct-to-patient contributes to the increase of study effectiveness and accelerates the publication of study endpoints and marketing authorization.
CRYOPDP Control Tower Solution - A “customer dedicated” end-to-end Control Management and Monitoring Service. The Control Tower ensures the premium execution of the customer predefined processes: from Order to Bill including booking, shipping, delivery. It provides a full supply chain visibility and real-time analytics, to guarantee total real time supervision of all movements at regional or global level, including pro-active incidents resolutions. The unique Global Control Tower Solution enhances CRYOPDP customers' existing logistics planning and execution systems, providing fully customizable solutions guaranteeing on time and under customer specifications performance above 99.9%.
MVE Biological Solutions Fusion Cryogenic Freezers - are the world’s first and only self-sustaining cryogenic freezer. The Fusion can operate as a stand-alone unit and requires no on-going liquid nitrogen supply or connection to and external liquid nitrogen source. Fusion freezers are a perfect solution for remote geographic locations, isolated laboratories, high elevation facilities, or facilities without existing liquid nitrogen infrastructure.
MVE Biological Solutions Vario Cryogenic Freezers - are innovative cryogenic freezers that can support temperatures anywhere between -20°C and -150°C. The Vario series of cryogenic freezers are able to provide effective and consistent temperature profiles with less than 1% of the power consumption and a 70% reduction in overall operating cost savings compared to traditional mechanical freezers.
In addition to these examples, Cryoport is continuously evaluating, expanding, and improving its range of services and solutions in response to the market requirements and client demand.
We intend to build on our history of developing market-leading, temperature-controlled supply chain solutions and delivering strong growth through the following strategies:
| ● | Superior service to our clients. We strive to provide our clients with best-in-class solutions to help manage some of the most difficult and critical aspects of their evolving businesses with advanced temperature-controlled supply chain solutions tailored to their specific requirements; |
| ● | Continuous innovation. We plan to capitalize on our internal technological expertise to develop products and solutions that address unmet needs in the global supply chain of the C> market and the other life sciences markets we serve. We plan to strengthen our existing products and solutions with complementary products, solutions and innovative technologies that are designed to provide our customers with tailored solutions to manage the critical aspects of the supply chain effectively and efficiently; |
| ● | Geographical expansion. We intend to expand our global commercial presence by continuing to broaden our capabilities within our existing network and selectively build out our new global supply chain center network, manufacturing facilities, and infrastructure in support of known and anticipated growth in demand for our solutions and equipment; |
9
| ● | Strategic logistics alliances and collaborations. We have been successful in establishing strategic alliances around the world as a means for our current and prospective client base to utilize our solutions. We have focused our efforts on market leading companies in supply chain services as well as participants in the life sciences industry. These strategies drive integration of our solutions into our alliance partners’ services. The overarching goal of these partnerships, through our Compliance Unified Ecosystem™, is to provide fully integrated solutions including, but not limited to, process optimization that reduces risk, increases transparency, and improves certainty; |
| ● | Targeted acquisitions. We intend to continue to selectively pursue acquisitions that may include innovative technologies and solutions and/or geographic competencies and capabilities to ensure we further enhance and broaden our market leadership and enable our clients to successfully bring products and life-saving therapies to market; and |
| ● | Setting industry standards. Our supply chain solutions are designed to support our clients’ initiatives through early-stage studies, clinical trials, and their global commercialization. We believe our ‘first mover’ advantage and the experience we have gained in supporting the cell and gene therapy market have positioned us as a market leader in the space with a strong platform of comprehensive solutions, products and services that have been adopted by many leading life sciences companies. A key strategy for further accelerating market adoption of our enabling solutions is to maintain and extend our position as the industry leader in the markets we serve. We believe this approach can enhance our support of the life sciences industry, further strengthen our market position, expand the breadth of our services, increase our competitive advantage and contribute to our long-term growth. |
The Markets We Serve
Cryoport serves the life sciences industry as a trusted provider of integrated temperature-controlled supply-chain solutions supporting the biopharma/pharma, animal health, and reproductive medicine markets.
Biopharma/Pharma. In the biopharma/pharma market, we are focused on supporting the saving of lives. From clinical research and development to clinical research organizations, to clinical trials for cell and gene therapies, to the storage and delivery of life-saving commercial cell and gene therapies, to the customers of biopharmaceutical and biotechnology organizations, to crucial points of care, we strive to address fundamental-to-advanced temperature-controlled storage, transport, packaging, fulfillment, and information challenges. Cell and gene therapies have become a rapidly growing area of biological drug development, with over $23.1 billion in funding raised in 2021 and 1,129 industry-sponsored, global clinical trials underway as of the third quarter of 2021, as reported by the Alliance for Regenerative Medicine in their 2022 Cell & Gene State of the Industry Briefing in January 2022. These therapeutic approaches have certain supply chain challenges that we believe our solutions are well tailored to address.
| ● | Cell Therapies. As per the Alliance for Regenerative Medicine, Cell therapy is “the administration of viable, often purified cells into a patient’s body to grow, replace, or repair damaged tissue for the treatment of a disease. A variety of different types of cells can be used in cell therapy, including hematopoietic (blood-forming) stem cells, skeletal muscle stem cells, neural stem cells, mesenchymal stem cells (adult stem cells that differentiate into structures as connective tissues, blood, lymphatics, bone, and cartilage), lymphocytes, dendritic cells, and pancreatic islet cells. Cell therapies may be autologous, meaning that the patient receives cells from their own body, or they may be allogenic, meaning the patient receives cells from a donor. Allogeneic cell therapies are often referred to as “off-the-shelf” therapies, as they are derived from a donor who is not the patient, enabling advance preparation and available to the patient immediately at the time of need.” |
Cryoport has been focused on the cell and gene therapy market for the last seven (7) years. In that time, we have successfully established ourselves as a premier provider of supply chain solutions and now support 602 clinical trials and 8 commercial programs in the space. Our solutions have been developed to specifically support this space and offer the highest level of temperature control and visibility within the market. Additionally, Cryoport has developed its Chain of Compliance™ platform that has been specifically developed to support irreplaceable clinical and commercial therapies and of which many of the critical requirements are featured in the recently released ISO 21973 guidance issued by the International Organization for Standardization (ISO) which specifically outlines General Requirement for Transportation of Cells for Therapeutic Use.
10
| ● | Gene Therapies. As per the Alliance for Regenerative Medicine, “Gene therapy seeks to modify or introduce genes into a patient’s body with the goal of durably treating, preventing, or potentially even curing disease, including several types of cancer, viral diseases, and inherited disorders.” These therapies often cost more than $1,000,000 per patient and are irreplaceable. Significant funding has been committed to this space with more than $10.6 billion in funding in 2021. Cryoport has developed its ELITE™ -80°C shipper line to support this space as a best-in-class product. |
Animal Health. In the animal health market, we provide support for animal reproduction, which primarily involves the production of protein for sustaining life. We also support medicine for the health of recreational and companion animals. Animal disease prevention and control rely on the safe transport and storage of vaccines and other biological materials around the world. Our temperature-controlled supply chain solutions are designed to help avoid costly delays through nonstop monitoring and complete fleet management from and to the origin and destination points as well as provide cryobiological storage equipment.
Reproductive Medicine. In the human reproductive medicine market, we are focused on the support of the creation of human life. This is primarily accomplished by supporting In Vitro Fertilization, or IVF, and related technologies along with clinical networks globally. Through our CryoStork® services, we transport reproductive materials through dedicated medical transport services to help ensure that IVF materials are on the next flight out to their destination. IVF materials also receive one-on-one handling and individualized attention during the entire logistics process. In addition, we also provide cryobiological storage equipment to fertility clinics around the world.
Acquisitions
We further expanded our capabilities in October 2020 by acquiring CRYOPDP, a leading global provider of innovative temperature-controlled logistics solutions for high value, time critical and temperature-sensitive pharmaceuticals. provides the biopharma market with temperature-controlled supply chain solutions including packaging, pick-pack kit preparation, premium services and specialty biopharma/pharma courier support. CRYOPDP added a network of 22 global logistics centers located in 12 countries to our global network. These additions expanded our logistics network giving us new advantages when serving global multi-national clients. They also added redundancies and backup that reduce supply chain risk for our clients. CRYOPDP is powered by a cloud-based logistics platform branded as UnITy™, which we plan to integrate with our Cryoportal® Logistics Management Platform. UnITy™, provides functionalities such as a transport management system, warehousing management system, quality management system, a customer experience portal, mobile apps for track and trace during transport and storage as well as integration with transportation agents and business partners. In the second quarter of 2021, we completed the acquisitions of Critical Transport Solutions Australia (CTSA) in Australia and F-airGate in Belgium to further enhance our existing global temperature-controlled supply chain capabilities in the APAC, and EMEA regions.
We also acquired MVE in October 2020. MVE provides cryobiological storage and transportation systems for the life sciences industry through its advanced line of cryogenic stainless-steel freezers, aluminum dewars and related ancillary equipment used in the storage and transport of life sciences commodities, which includes the rapidly growing cell and gene therapy business. With three primary facilities, located in Ball Ground, Georgia, New Prague, Minnesota and Cheng-du, China, MVE Biological Solutions is the leader in serving the life sciences industry throughout the world. The acquisition is a vertical integration that, in addition to expanding our footprint to handle the growing demand driven by the growth in the cell and gene therapy market, assures our supply of cryogenic systems. MVE strengthens Cryoport’s presence in all its markets, biopharma/pharma, animal health and reproductive medicine. Its clients include cell and gene therapy, medical laboratories, biotech/pharmaceutical research facilities, blood and tissue banks, animal breeders, veterinary laboratories, large-scale biorepositories, fertility clinics, etc.
In May 2019, we acquired Cryogene Labs (CRYOGENE), which is today an expanding state-of-the-art temperature-controlled biostorage solutions business strategically located in Houston, Texas. CRYOGENE is an industry leader in the management of pre-clinical biostorage services which include critical biological commodities supporting clinical research, the advancement of cell and gene therapy, and public health research. It provides customized, end-to-end chain of custody/chain of condition solutions for its clients. CRYOGENE is a FDA audited operation serving all temperature categories of the cold chain for the life sciences.
As of December 31, 2021, we lease or own 33 total sites across the Americas, EMEA and APAC regions.
11
Segment Reporting
The Company continually monitors and reviews its segment reporting structure in accordance with authoritative guidance to determine whether any changes have occurred that would impact its reportable operating segments. Operating segments are defined as components of an enterprise about which separate financial information is available that is evaluated regularly by the chief operating decision maker in deciding how to allocate resources and in assessing operating performance. The chief operating decision maker (“CODM”) is our Chief Executive Officer. Up until the fourth quarter of 2020, we managed, reported and evaluated our business in the following two reportable operating segments: Global Logistics Solutions and Global Bioservices. During the fourth quarter of 2020, our CODM changed how he makes operating decisions, assesses the performance of the business and allocates resources in a manner that caused our operating segments to change as a result of the MVE Biological Solutions and CRYOPDP acquisitions. In consideration of Financial Accounting Standards Board's ("FASB") Accounting Standards Codification ("ASC"), Segment Reporting, we determined that we are not organized around specific products and services, geographic regions or regulatory environments. Accordingly, beginning with the fourth quarter of 2020 we realigned our reporting structure, resulting in a single reportable segment. The Company has adjusted its financial statements for historical periods to reflect this change in segment reporting and show its financial results without segments for all periods presented. See Part II, Item 7 – Management's Discussion and Analysis of Financial Condition and Results of Operations, in this Annual Report on Form 10-K for further discussion.
Customers and Distribution
As a result of growing globalization, including in such areas as biologics, biopharma, biotechnology, clinical trials, distribution of biopharmaceutical products and reproductive medicine, the requirement for effective and reliable solutions for keeping clinical samples, pharmaceutical products and other specimen at controlled temperatures takes on added significance due to more sophisticated supply chain challenges including complex shipping routes, extended shipping times, potential custom delays, general logistics challenges, distribution and storage requirements. We believe that our platform of solutions, expertise and geographic footprint enables us to take advantage of the growing demand for effective and efficient international transport and storage of temperature sensitive life sciences commodities and products. This is especially the case for the new therapies being developed in the regenerative medicine market, such as autologous and allogeneic CAR-T cell therapies, that require cryogenic temperatures to maintain safety and efficacy.
No single customer accounted for over 10% of our total revenues during the years ended December 31, 2021 and 2020. Two customers accounted for 24.1% and 12.8% of our total revenues during the year ended December 31, 2019.
Our geographical revenues, by origin, for the years ended December 31, 2021, 2020 and 2019, were as follows:
|
2021 |
|
2020 |
2019 |
|
||
Americas |
|
54.0 |
% |
63.0 |
% |
84.9 |
% |
Europe, the Middle East and Africa (EMEA) |
|
26.7 |
% |
25.8 |
% |
13.3 |
% |
Asia Pacific (APAC) |
|
19.3 |
% |
11.2 |
% |
1.8 |
% |
12
Customer types
Cryoport is a global leader in serving the life sciences industry as a trusted provider of integrated temperature-controlled supply-chain solutions supporting the biopharma/pharma, animal health, and reproductive medicine markets. Our major customer types include:
Clinical Trials - Every pharmaceutical or biotech company developing a new drug or therapy must seek development protocol approval by regulatory bodies, e.g., the FDA or EMA. Usually, these agencies require clinical trials to be designed to test the safety and efficacy of the potential new drug or therapy among other things. In connection with the clinical trials, due to globalization, companies may enroll patients from all over the world and may need to regularly submit a blood or other specimen at the local hospital, doctor’s offices or laboratories. Generally, these samples are then sent to specified testing laboratories, which may be local or in another country. In addition, the therapies used by the patients may require biostorage and time critical and/or temperature-controlled shipping to the sites of the clinical trials. Importantly, clinical trial specimens are often irreplaceable because each one represents clinical data at a prescribed point in time, in a series of specimens on a given patient, who may be participating in a trial for years. Sample integrity during the logistics process is vital to retaining the maximum number of patients in each trial and keeping the trial on schedule. Requirements can also include the return and destruction of these investigational medicinal products. Clinical trial support involves a full ecosystem of Clinical Research Organizations (“CRO”) and requires flexible and highly qualified end-to-end supply chain solutions for which we ideally suited.
Biotechnology and Diagnostic Companies - The biotechnology market includes basic and applied research and development in diverse areas such as stem cells, gene therapy, DNA tumor vaccines, tissue engineering, genomics, blood products, etc. Companies participating in the foregoing fields rely on the frozen storage and temperature-controlled transport of specimens in connection with their research and development efforts, for which our suite of supply chain solutions are ideally suited.
Cell Therapy Companies - Rapid advancements are underway in the research and development of cell-based therapies, which involve cellular material being infused into a patient. In allogeneic cell therapies, the donor is a different person than the recipient of the cells. Autologous cell therapy is a personalized therapeutic intervention that uses an individual’s cells, which are cultured and expanded outside the body, and reintroduced into the individual. Once cells are manufactured into a cellular therapy, in either case, they must be stored and shipped cryogenically for which our Cryoport Express® and Cryoport ELITE™ Shipper solutions, CRYOPDP solutions, CRYOGENE biostorge, and MVE Biological Solutions’ stainless steel cryogenic freezers and aluminum dewars are ideally suited.
Contract Research, Development & Manufacturing Companies - Increasingly, as evidenced by our strategic partnership with Lonza, CRO’s, and Contract Development and Manufacturing Organizations (“CDMO’s”) are engaging our services exclusively in conjunction with their contract services platform in order to provide a higher level of service to our mutual client base. We anticipate that these relationships, which are mutually beneficial to both parties as well as our client base, will accelerate and expand to include our entire portfolio of services as cell and gene therapy clinical trials advances and as commercial therapies ramp on a global basis.
Central Laboratories - With the increase and globalization of clinical studies and trials, logistics have become more complex and ensuring sample integrity has become more challenging. International courier costs are now consuming an increasing portion of global protocol budgets; however, central laboratories are also looking for reliability, thus we believe laboratories performing the testing of samples collected during the conduct of these global multi-site studies are looking for cost effective, reliable state-of-the-art supply chain solutions. CRYOPDP’s global network of logistic centers have successfully supported central laboratories throughout the world for many years.
Pharmaceutical or Therapy Distribution - The current focus for our products and solutions also includes the area of bio-pharmaceutical supply chain. There are a significant number of therapeutic therapies currently or anticipated soon to be undergoing clinical trials. After the FDA or regional or national authorities in EMEA or APAC approve them for commercial marketing, it will be necessary for the manufacturers to have a reliable and economical method of bio-storage and distribution to the physician who will administer the product to the patient. We believe it is likely that the most efficient and reliable method of distribution will be to ship a single dosage to the administering physician. These therapies are typically identified to individual patients and therefore will require a complete tracking history from the manufacturer to the patient. The most reliable method of doing this is to ship a unit dosage specifically for each patient. If such therapies require temperature-controlled logistics, we can provide the technology to meet this need. This supply chain management also includes the support of Managed Access Programs, whereby patients with serious diseases sometimes require specific therapies that are not yet available and/or approved by local regulatory authorities.
13
Fertility Clinics and IVF. Maintaining cryogenic temperatures during shipping and transfer of in vitro fertilization specimens like eggs, sperm, or embryos is critical for cell integrity to retain viability, stabilize the cells, and ensure reproducible results and successful IVF treatment. We believe that Cryoport Systems solutions for this market, branded as CryoStork® services, are very compelling and well received. Additionally, MVE supplies stainless steel cryogenic freezers to fertility clinics that wish to store reproductive materials on site. The Assisted Reproductive Technology (“ART”) industry is also starting to undergo a significant change due to the consolidation of clinic networks into large corporations or venture backed organizations, which we believe will allow us to further build out our leadership position and set industry standards.
Animal Health Companies. The global animal health market is largely driven by a significant rise in the zoonotic and food-borne diseases globally. This unprecedented disease prevalence has encouraged companies to produce advanced vaccines and pharmaceuticals. The high demand has also resulted in the subsequent rise in the number of companies making consistent efforts to control risks of pathogen contamination and food-borne diseases, which is contributing to market growth. In addition to food animal production, companion animal support is another emerging area in which companies are investing heavily. Cryoport’s MVE products, Cryoport Systems and CRYOPDP temperature-controlled supply chain solutions are well positioned to support storage and distribution needs of breeders, large-scale repositories, veterinary laboratories, and artificial insemination, particularly in the beef and dairy industries on a global basis.
University Research Facilities. Research is conducted globally at major universities. Often this is done in collaboration with others which requires shipping using Cryoport Express® Shippers or CRYOPDP services. When in storage, we believe storage at cryogenic temperatures provides the most secure long-term storage for high value samples. Our broad line of products provides solutions tailored to institutions and individual researchers as exemplified by CRYOGENE.
Sales and Marketing
We serve clients throughout the life sciences industries and our sales and marketing initiatives are global in nature, focusing on addressing each customer’s pain points and anticipated needs through best-in-class supply chain solutions. Our business development teams have resources in the Americas, EMEA and the APAC regions to proactively support our customers and to ensure efficient business development with respect to the customer’s geographic activity, size and decision-making structure. Our marketing teams design and implement targeted digital campaigns to support our commercial strategy and promote our innovative portfolio of solutions and capabilities. Our marketing initiatives are designed to drive our business development, program management, consulting, other related activities and increase awareness of our advanced temperature-controlled supply chain solutions. Given the global nature of our business and the high focus on customer development and acquisition, we plan to continue to extend our sales and marketing team’s reach in the Americas, EMEA and the APAC regions by further strengthening our marketing initiatives, hiring additional resources, and expanding our distributor and alliance network.
Competition
We believe Cryoport is unique in its offering, and we have not identified any competition that offers solutions that are as comprehensive or as in depth as our platform of temperature-controlled supply chain solutions and that has been proven in the global market to the same extent as our solutions have. However, we do have competition from companies that offer products and/or services that could be considered competitive to certain components or elements of our platform of solutions include specialty couriers, such as World Courier Group, Inc., Marken, Biocair and Quick Life Science Group along with companies that offer products such as Biolife Solutions, Azenta Life Sciences, Cytiva, and IC Biomedical. In addition, life science companies may develop their own in-house temperature-controlled supply chain solutions, systems and procedures to cover their needs.
14
Engineering and Development
Our research, development and engineering efforts are focused on continually investigating new technologies that can improve our services and improving the features of our products and solutions, which includes our cloud-based Cryoportal® Logistics Management Platform, Cryoport Express® and Cryoport ELITE™ Shippers, secondary packaging solutions, our SmartPak condition monitoring system, technology used to enhance our specialty courier solutions, as well as our advanced line of stainless-steel freezers, aluminum dewars and related ancillary equipment used in the storage and transport of life sciences commodities. These efforts are expected to lead to, based on market requirements, the introduction of additional systems and features, including dewars and other shippers of varying sizes and for various temperature ranges, bio-storage units of varying sizes and for varying ranges, further advanced informatics, and improved monitoring systems. We are continuously researching alternative and new technologies, lower cost materials, utilization of higher volume assembly methods, improved manufacturing methods and enabling technologies that will make it practical to provide a wider range of effective and advanced solutions. Some of these developments are further described under ‘Cryoport Products and Services’ further above.
Innovation
Supporting critical supply chain demands within the cell therapy space requires unique, dedicated temperature-controlled systems and processes. Applying traditional logistics methodology in support of cell therapies is often ineffective. Cryoport continues to develop a dedicated supply-chain platform specifically tailored to cell therapy storage, fulfillment, and distribution requirements. It is anchored by our Chain of Compliance® ISO 21973 compliant platform and supported by industry leading processes, technology, and experience. Critical to the advancement of our supply-chain platform is the ability to develop and launch market leading technologies, systems, and processes that continually improve the management of risk, provide the ability to scale, and enable our clients to focus on the development and manufacturing of their critical therapies and treatments. Specific examples that we have developed and/or have recently launched or are launching include the following novel supply chain products and services in support of our current offerings.
Cryoport ELITE™ Shipper Systems
Cryoport ELITE™ -80°C Gene Therapy Shipper. As the first product in a high-performance line of Cryoport ELITE™ Shippers, the company has designed a best-in-class family of -80°C shippers that have superior temperature management properties as well as incorporate next generation protection, handling, and data collection and management systems including our SmartPak™ Condition Monitoring System. The Cryoport ELITE™ shipper line has been developed in conjunction with one of the leaders in the gene therapy space for clinical and commercial gene therapy distribution. The ELITE shipper platform will be launched in 1H 2022.
Cryoport ELITE™ Cryosphere™ Shipper. The second product in the new high-performance line of Cryoport ELITE™ Shippers is the Cryosphere™, which is a revolutionary gravitationally stabilized shipper that is the most advanced cryogenic shipper to support the Cell and Gene Therapy and other life sciences markets. The shipper is designed to passively stabilize the payload through an internal gravitational sphere, thereby keeping the payload in an upright orientation regardless of the external shipper orientation. This innovative technology further mitigates one of the key risks during storage, handling, and transport, which is maintaining constant cryogenic temperatures. In addition, the Cryosphere™ will have advanced shock and vibration absorption properties to further protect the payload and will be outfitted with Cryoport’s state-of-the-art condition monitoring systems. It has also been designed to be ergonomically friendly for our manufacturing and clinical partners providing a better user experience than other products in the market. The Cryosphere™ is expected to be launched during the first half of 2022.
MVE Fusion®
MVE Fusion Cryogenic Freezers are the world’s first and only self-sustaining cryogenic freezer. The Fusion is able to operate as a stand-alone unit and requires no on-going liquid nitrogen supply or connection to and external liquid nitrogen source. Fusion freezers are a perfect solution for remote geographic locations, isolated laboratories, high elevation facilities, or facilities without existing liquid nitrogen infrastructure.
15
MVE Vario®
MVE Vario Cryogenic Freezers are innovative cryogenic freezers that can support temperatures anywhere between -20°C and -150°C. The Vario series of cryogenic freezers are able to provide effective and consistent temperature profiles with less than 1% of the power consumption and a 70% reduction in overall operating cost savings a compared to traditional mechanical freezers.
Cryoport Data Management Systems
SkyTrax™ Condition Monitoring System. SkyTrax™ is a next generation Condition Monitoring System. SkyTrax™ will be the only proprietary designed condition monitoring system custom built for the cell and gene industry. In addition to being 4G/LTE compliant with a 3G/2G back-up, cellular network agnostic, with a full sensor array to track location, temperature, humidity, light, shock, orientation, and geofencing, it will be Bluetooth and Wi-Fi capable, have triple data redundancy, a best-in-class battery life, as well as bi-directional communication providing scannable airway bills, commercial invoices, loading and unloading instructions, security access features, as well as temperature data via an E-ink screen. Upon introduction in 2022, it will also have the ability to be reprogrammable remotely in support of critical in-field logistics needs. SkyTrax™ will be fully integrated into our Cryoport Express® and Cryoport ELITE™ Shipper Systems.
Cryoportal® 2.0 and UnITy™. Cryoport Systems’ Cryoportal® 2.0. Logistics Management Platforms the industry’s only ISO 21973 compliant supply chain management platform. In addition to managing all aspects of a given client shipment, it also manages all elements of the Chain of Compliance™ based aspects of the packaging as well including shipper management, requalification, and processing. Cryoport Systems’ Cryoportal® 2.0 is complimented by CRYOPDP’s recently released UnITy™ Transportation Management System. UnITy™ provides functionalities in addition to transport management that include: warehousing management, quality management, a Customer Experience portal, mobile apps for track and trace during transport and storage as well as integration with transportation agents and business partners. The combination of these two powerful informatics platforms provides Cryoport clients with a comprehensive status of their clinical or commercial distribution activities, supports regulatory requirements and further sets Cryoport apart from competition.
Global Supply Chain Centers
Cryoport Global Supply Chain Center Network. During the first half of 2022, Cryoport will launch its first two cell and gene therapy purpose-built Global Supply Chain Centers in Houston, Texas and Morris Plains, New Jersey. Each of these global facilities in addition to supporting critical logistics activities will also support all kitting, fulfillment, storage, secondary labelling, and drug return and destruction needs for our clients. They are specifically tailored to the needs of both autologous and allogeneic cell therapies, gene therapies, RNA therapies, and vaccines. We believe these first of their kind global supply chain centers are the beginning of a distinguishing global network that will offer unique advantages to the advancement of the regenerative medicine market.
Manufacturing and Raw Materials
Manufacturing. We source components for our products from multiple suppliers, including those that manufacture to our engineering specifications, using, in part, proprietary technology and know-how to mitigate supply chain risks. We also use “off-the-shelf” products, which we may modify to meet our requirements. For some components, there are relatively few alternate sources of supply and the establishment of additional or replacement suppliers may or may not be accomplished immediately. When this occurs, we endeavor to mitigate risk by locating an alternative qualified supplier and, as appropriate, increasing our inventory level. For additional information see the “Risk Factors” section of this document.
Our vendor/partner relationships allow us to concentrate on further advancing and expanding our platform of systems, products, and solutions for the life sciences to meet the growing and varied demands for validated temperature-controlled solutions in the life sciences industry. We endeavor to keep our supply structure up to date and agile as it provides us the opportunity to rapidly scale to support our client’s commercialization, systems, products, and solutions requirements; however, we are ever mindful of the work we must do to improve our current sourcing and to continue to mitigate risks therein.
Raw Materials. Various raw materials are used in the manufacture of our products and in the development of our technologies. Most raw materials are generally available from several alternate distributors and/or manufacturers. Where we have experienced significant difficulty in obtaining these raw materials, we have established alternative global sources or are working with the existing supplier to overcome its deficiency.
16
Patents, Copyrights, Trademarks and Proprietary Rights
To remain competitive, we must develop and maintain protection on the proprietary aspects of our platform of technologies. We rely on a combination of patents, copyrights, trademarks, trade secret laws and confidentiality agreements to protect our intellectual property rights.
We file patent applications to protect innovations arising from our research, development and design. We currently own approximately 32 issued patents and have more than 50 pending patent applications throughout the world. Our patents generally protect certain aspects of our products and related technology. We also own common law and registered trademarks in the U.S. and in certain foreign countries to protect the names of our company, certain products, and key service brands. We own certain copyrights relating to certain aspects of our systems, products and services.
Our success is influenced, in part, by our ability to continue to develop proprietary products and technologies. It is desirable to obtain patent coverage for these products and technologies; however, some are protected as trade secrets. We intend to file trademark and patent applications covering any newly developed products, methods and technologies. However, there can be no guarantee that any of our pending or future filed applications will be issued as patents or registered as trademarks. There can be no guarantee that the various patent and trademark governmental agencies from around the world or some third party will not initiate an interference proceeding involving any of our pending applications or issued patents. Finally, there can be no guarantee that our issued patents or future issued patents, if any, will provide adequate protection from competition.
Patents provide some degree of protection for our proprietary technology. However, the pursuit and assertion of patent rights involve complex legal and factual determinations and, therefore, are characterized by significant uncertainty. In addition, the laws governing patent issuance and the scope of patent coverage continue to evolve. Moreover, the patent rights we possess or are pursuing generally cover our technologies to varying degrees. As a result, we cannot ensure that patents will issue from any of our patent applications, or that any of the issued patents will offer meaningful protection. In addition, our issued patents may be successfully challenged, invalidated, circumvented, or rendered unenforceable so that our patent rights may not create an effective barrier to competition. We must also pay maintenance fees at set intervals for our patents to not expire prematurely. The laws of some foreign countries may not protect our proprietary rights to the same extent as the laws of the United States. There can be no assurance that any patents issued to us will provide a legal basis for establishing an exclusive market for our products or provide us with any competitive advantages, or that patents of others will not have an adverse effect on our ability to do business or to continue to use our technologies freely. As with all patents, we may be subject to third parties filing claims that our technologies or products infringe on their intellectual property. We cannot predict whether third parties will assert such claims against us or whether those claims will hurt our business. If we are forced to defend against such claims, regardless of their merit, we may face costly litigation and diversion of management’s attention and resources. As a result of any such disputes, we may have to develop, at a substantial cost, non-infringing technology or enter into licensing agreements. These agreements may be unavailable on terms acceptable to such third parties, or at all, which could seriously harm our business or financial condition.
With respect to our trademarks, we file and pursue trademark registrations on words, symbols, logos, and other source identifiers that clients use to associate our products and services with us. Although our registered trademarks carry a presumption of validity, they can be challenged and possibly invalidated and as such, we cannot guarantee that any trademark registration is infallible.
We also rely on trade secret protection of our intellectual property. We attempt to protect trade secrets by entering into confidentiality agreements with employees, consultants and third parties, although, in the past, we have not always obtained such agreements. It is possible that these agreements may be breached, invalidated, or rendered unenforceable, and if so, our trade secrets could be disclosed to our competitors. Despite the measures we have taken to protect our intellectual property, parties to such agreements may breach confidentiality provisions in our contracts or infringe or misappropriate our patents, copyrights, trademarks, trade secrets and other proprietary rights. In addition, third parties may independently discover or invent competitive technologies, or reverse engineer our trade secrets or other technology. Therefore, the measures we are taking to protect our proprietary technology may not be adequate.
17
Cryoport’s Quality Assurance and Regulatory Affairs Programs
Cryoport is committed to quality, and this is reflected in all aspects of our global organization. From our innovative design of products and services to our continuous improvement initiatives, Cryoport has implemented comprehensive quality standards that match or exceed the stringent requirements within the markets we serve. Cryoport’s Quality Management Systems have been designed, implemented, and certified to meet ISO 9001:2015 and ISO 13485 standards in key global locations, demonstrating the discipline necessary to maintain a positive compliance profile. With our strong foundation in ISO 9001:2015 and ISO 13485, we leverage industry-specific experience with applicable regulatory requirements, and industry expectations, to create processes and procedures that incorporate strong operational practices of checks with verification. Our Quality Management Systems are designed to ensure proper controls in logistics, customer/client education, contracting, processing, shipping and storage, accumulation, and communication. Providing each customer with the proper information, monitoring, and data collection to support Chain of Compliance, Chain of Custody and Chain of Condition, culminating in the successful completion of each transaction or shipment within regulatory requirements.
Our Quality Management Systems incorporate notable good practice quality guidelines and regulations (GxP) elements, beyond those stipulated in ISO 9001:2015 and ISO 13485, to ensure our customers are supported in the manner necessary to maintain standards and to secure a positive compliance profile for Cryoport as a supplier and partner. Notable elements include, but are not limited to, Good Documentation Practices, Good Distribution Practices, Archival processes and procedures, Supplier Controls, and Corrective Action and Preventive Action (CAPA) procedures, to highlight a few examples.
Through procedural requirements, Cryoport provides substantial risk-mitigation strategies throughout its full offering of products, systems, and services to support and maintain customer confidence. Metrics and Key Performance Indicators are accumulated regularly, and are trended to predict, and mitigate, potential risks to operations. Senior management utilized this information to enact decisions regarding procedures, processes, resource allocation, and corrective actions. Quality-driven initiatives are supported throughout our global organization. In addition, Cryoport’s manufacturing operations are ISO 13485 certified as medical manufacturing facilities and are audited for compliance on a regular basis by authorized authorities. We are also subject to GMED, which is an international reference body in the certification of health care and medical devices quality management systems under ISO 9001, NF EN ISO 13485 and ISO 13485. As such, we are subject to audits buy a Medical Device Single Audit Program (MDSAP) auditing organization. Cryoport’s cryogenic storage facilities are routinely inspected by the U.S. Food and Drug Administration (FDA) and The Foundation for the Accreditation of Cellular Therapy (FACT) to confirm regulatory compliance to industry requirements related to drug applications, filings, and maintenance of various cryogenically stored materials.
Government Regulation
Globally, Cryoport is subject to numerous domestic federal, state, and local laws and regulations as well as international regulations relating to shipments, customs, import, export, safe working conditions, manufacturing practices, environmental protection, and disposal of hazardous or potentially hazardous substances. In addition, we must ensure compliance with economic sanctions and/or restrictions on individuals, corporations, or countries, and other government regulations affecting trade that may apply to our international cross border business activities.
The shipping of biologic products, biologic commodities, diagnostic specimens, infectious substances, and dangerous goods, whether via air or ground, falls under the jurisdiction of many state, federal and international agencies. The quality of the packaging that protects such entities is critical in determining successful shipping conditions and to ensure a commodity will arrive at its destination in a satisfactory condition. Meeting stringent regulations such as Dangerous Goods Regulations, ISTA, and IATA, as applicable, Cryoport has demonstrated compliance and adhesion to these requirements. Many of the regulations for transporting dangerous goods in the United States are determined by international rules formulated under the auspices of the United Nations. Dangerous goods are typically one-time shipments and are not a part of our routine services. When called upon to ship dangerous goods, Cryoport follows strict and stringent guidelines. International Civil Aviation Organization (“ICAO”) is the United Nations organization that develops regulations (Technical Instructions) for the safe transport of dangerous goods by air. If shipment is by air, compliance with the rules established by the International Air Transport Association (“IATA”) is required. IATA is a trade association made up of airlines and air cargo couriers that publishes annual editions of the IATA Dangerous Goods Regulations. These regulations interpret and add to the ICAO Technical Instructions to reflect industry practices. Additionally, the Centers for Disease Control (“CDC”) has regulations (published in the Code of Federal Regulations) for interstate shipping of specimens.
18
Our Cryoport Express® and ELITE™ Shippers meet Packing Instructions 602 and 650 and are certified for the shipment of Class 6.2 Dangerous Goods per the requirements of the ICAO Technical Instructions for the Safe Transport of Dangerous Goods by Air and IATA. Our present and planned future versions of the Cryoport SmartPak™ Condition Monitoring Systems will likely be subject to regulation by the Federal Aviation Administration (“FAA”), Federal Communications Commission (“FCC”), Food and Drug Administration (“FDA”), IATA and possibly other agencies which may be difficult to determine on a global basis. Additionally, our Chain of Compliance™ processes comply fully with ISO 21973 recommendations.
Storage of biological materials that are classified as drug products for human therapeutic use (either for investigational use or commercially approved) or materials used in the manufacture of drug products for human therapeutic use, is regulated by the FDA under Title 21 Code of Federal Regulations (“CFR”) part 210 & 211. Facilities must be compliant with current Good Manufacturing Practice (“GMP”) regulations which are enforced by the FDA through registration and audit. When drug products are exported to other countries, storage upon receipt must meet relevant local regulations.
Our MVE Biological Solutions cryogenic stainless-steel freezers and aluminum dewars are certified to the Medical Device Directive (MDD) in the European Union. Additionally, registrations for import are in place for various countries with these requirements.
For additional information, See “Part I, Item 1A — Risk Factors—Risks Related to Regulatory and Legal Matters” in this Form 10-K.
Environmental, Social and Governance (“ESG”)
Beginning in 2020 we initiated a formal internal evaluation of our ESG policies, procedures, and performance. Subsequently in February 2021, we publicly disclosed ESG information based on the framework and standards set by the Sustainability Accounting Standards Board (SASB) and the Taskforce on Climate-related Financial Disclosures (TCFD). Building upon our first report, we began with the goal of developing a formal, thoughtful, comprehensive, and right-sized sustainability program that would be used as a foundation for effectively organizing, reporting, and measuring our performance to set ESG goals in the future.
In June of 2021, we began a materiality assessment to guide our overall sustainability strategy. The intent of the materiality assessment was to understand what ESG topics were important to our key stakeholders, to take into consideration Cryoport’s business strategy development, and to understand Cryoport’s global internal priorities. There were three key activities for this phase of the process:
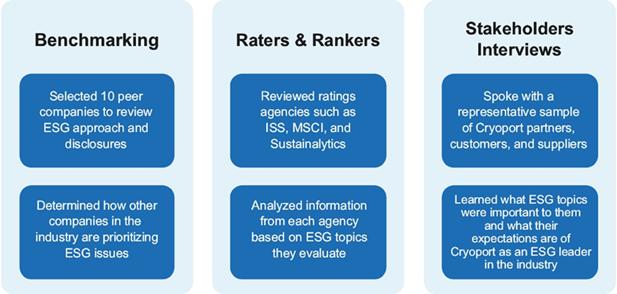
The information and feedback received from the materiality assessment was aggregated into a customized and weighted materiality matrix. The following Materiality Matrix follows GRI Standards recommendations and plots topics based on their relative priority resulting from the materiality assessment.
19
Once the Materiality Matrix was developed, several meetings were conducted internally with our ESG committee and our Board of Directors’ Nomination and Governance Committee to evaluate the findings.
Cryoport Materiality Matrix
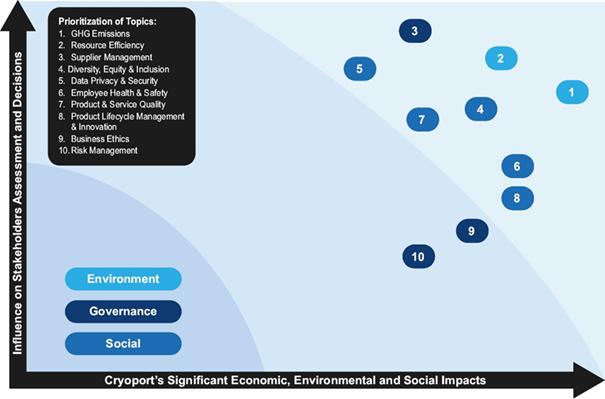
20
Leveraging the outcomes of the materiality assessment, Cryoport’s Sustainability Strategy was developed to establish focus and a consistent approach to sustainability across the business. Cryoport’s Sustainability Framework defines our overarching vision and mission statements with supporting pillars and corresponding focus areas, as demonstrated below.
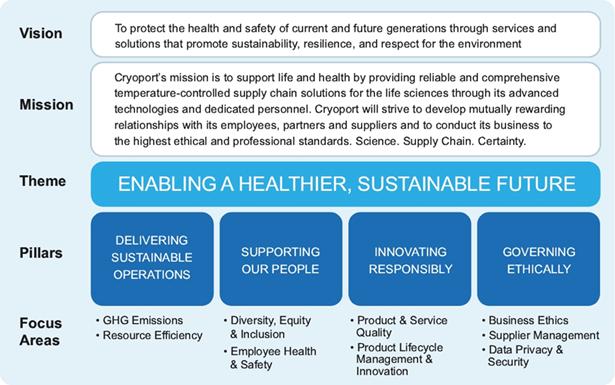
Environmental
As Cryoport continues to grow its business in a way that is considerate of our global community, we are committed to protecting our planet by using our world’s resources sensibly and minimizing our emissions and waste on a global basis. From an environmental standpoint, one example of our sustainability efforts is our cryogenic Cryoport Express® Shipper, which uses the non-hazardous dry vapor form of liquid nitrogen and proprietary informatics to drive efficiencies in the use of resources throughout our company. This service offering also employs multi-use and recyclable packaging. Knowing that there is much more to do to aid in our environmental efforts, we have recently developed a system to collect data on a global scale for the purpose of quantifying the impact of all our environmental initiatives so that we can demonstrate our achievements on this important matter to our shareholders, customers and other interested stakeholders.
Greenhouse Gas Emissions (GHGs)
As we proceed on our ESG journey in 2022, our initial key focus will be on GHG Emissions. GHG emissions were the foremost priority identified in our Materiality Matrix and represent a clear global significance for companies, consumers, and other stakeholders.
21
Cryoport engaged an ESG advisor upon completion of our Sustainability Strategy to assist in determing our carbon footprint. Through this engagement, we will define our Scope 1 and Scope 2 emission sources, collect and compile relevant data to quantify our emissions, and establish a carbon accounting methodology in accordance with the GHG Protocol. The outcome will be a baseline carbon footprint for Cryoport and the ability to execute consistent carbon accounting in the future. These tools will enable us to identify key opportunities to implement targeted projects that will drive GHG emissions reductions in the future. One means of reducing our carbon footprint is procurement of electricity from GHG emissions free resources for our biostorage plant in Houston, Texas. Procurement of GHG emissions free electricity has translated to a significant reduction in carbon emissions compared to energy provided by coal-fired power plants. In 2021, the Houston plant consumed 4,489,987 kWh of electricity. By utilizing electricity from GHG emissions free resources, the emission of 1,440,257 pounds of GHGs was avoided. This emissions savings is the equivalent of removing 242 passenger vehicles driven for one year.
Resource Efficiency
Cryoport understands the importance of resource conservation as it relates to the efficient use of resources, reduction of our carbon footprint, and effective cost management. To that end, Cryoport strives to operate in an efficient manner to ensure the optimization of raw materials, equipment, waste, energy and labor. We have identified thermal efficiency as the largest impact on energy conservation associated with our products. To manage this aspect of the business, our Global Logistics Center Network utilizes the International Safe Transit Association (ISTA) standard 7E to test and evaluate thermal performance against stated requirements of 10+ days of maintaining internal temperatures at or below negative 150oC. Our products comply with this standard, and in turn, the products insulative properties require less energy to maintain prescribed temperature levels.
Cryoport’s dewars do not consume any electricity, and our freezer units utilize liquid nitrogen as a cooling agent, which is the by-product of other processes, creating a closed-loop resource that reduces additional energy associated with sourcing, procuring, and delivery of resources purchased from third parties. In 2021, our use of liquid nitrogen freezers, when compared to the energy consumption of a standard -150OC mechanical freezer, prevented the consumption of 1,883,136 kWh of electricity, equivalent to 1,725,871 pounds of GHG emissions avoided.
We also exemplify efficiency through the development and use of the cryogenic Cryoport Express® Shipper, which uses the non-hazardous dry vapor form of liquid nitrogen and proprietary informatics to drive efficiencies in the use of resources throughout our company. Our Cryoport Express® Shipper is also a multi-use offering with recyclable packaging.
In addition, Cryoport measures the effectiveness and efficiency of our production practices by tracking the amount of scrap material disposed of or sold on an annual basis. Stainless steel products that do not meet our stringent specification are reworked into our process to create new products that meet specifications, diverting the material from landfill and scrap recycling for beneficial reuse as new product. Any scrap metal that does not meet specification and cannot effectively be reworked into new product is recycled by third party recycling partners.
Further, in 2021 our Paris, France operations moved into a new facility, which is designed with the highest French environmental standards and equipped with solar panels to reduce energy consumption and GHG emissions. Overall, our efforts in these areas, among others not highlighted, have a positive environmental outcome for Cryoport and its customers.
Supporting Our People
Cryoport’s global team of employees are our most valuable resource, from our teams on the front line in our global supply chain and logistics centers, to our manufacturing operations, to our business development personnel, to the engineers who design our products and services, to our quality assurance and regulatory teams that assure the safety, quality, compliance, and integrity of our products.
Diversity, Equity & Inclusion (DEI)
We are committed to inclusion, equity and diverse representation for our employees across our Company. Cryoport is an Equal Employment Opportunity employer and currently tracks gender distribution across its operations and management. We maintain clear policies related to anti-harassment, discrimination and retaliation, and provide an anonymous, third party-managed reporting hotline for employees to report incidents of harassment, discrimination, and policy violations. In 2021, we further enhanced our online corporate training programs related to diversity, harassment and discrimination, so that employees receive annual training on the topics of
22
harassment, diversity and inclusion, business ethics and code of conduct. In addition, Cryoport’s recruiting programs include targeted outreach to a variety of under-represented constituents, including minorities, women, veterans, and disabled populations to help improve recruiting efforts while gaining valuable insights from a diverse set of recruits. Cryoport has partnered with or targeted organizations like Hire Heroes, Career OneStop, recruiting at Historical Black Colleges, Accounting and Financial Women’s Alliance, and Women in Technology.
Cryoport understands that the manufacturing industry is typically male-dominated. In an effort to open opportunities for more female roles within our organization, Cryoport currently tracks gender distribution across operations. As of December 2021, women represented a total of approximately 30% of all employees, with approximately 18% of manager and 8% of directors being women. Senior leadership positions, at Vice President and above, currently sits as approximately 2%. Based on this data, Cryoport understands that there is work to be done to create a more equitable and representative senior leadership team and continue to push gender diversity throughout its operations.
Employee Health & Safety
Cryoport’s Employee Health & Safety (EHS) programs have resulted in strong safety performance, as demonstrated by our total injury rate (TIR) and lost time injury rate (LTIR) being significantly lower than the global industry averages. Facilitated by our culture of continuous improvement, we are committed to continue to work toward reducing our TIR and LTIR numbers even further.
We have implemented flexible working arrangements, including telecommuting and part time arrangements, in order to maintain a safe working environment for our employees throughout the COVID-19 pandemic.
Innovating Responsibly
Cryoport recognizes the role we play in protecting the health and safety of current and future generations through services and solutions that promote sustainability, resilience, and respect for the environment. We strive for a product base that is of the highest quality and with long use phases to minimize impact associated with production of new product, and Cryoport reviews opportunities to eliminate materials of concern and related managed waste streams on a regular cadence.
Product & Service Quality
As a temperature-controlled supply chain provider to the life sciences industry, Cryoport must comply with the safe transportation of regulated hazardous materials. As a result, we have designed and developed several features in its various products to comply with US DOT, IATA, ICAO, and other regulatory and guidance bodies. Additionally, safety warnings are included in our product labeling as well as our manuals. Our products are designed to conform to the following standards (where applicable):
| ● | ISO 13485 (Section 7.3 Design and Development, ISO, QMS) |
| ● | ISO 14971 Application of Risk Management, ISO |
| ● | Medical Device Directive Medical Devices Directive 93/42/EEC, and Directive 2007/47/EC amending Council Directive 93/42/EEC concerning medical devices |
| ● | Low Voltage Directive (LVD) (2014/35/EU) |
| ● | Electromagnetic Compatibility Directive (2014/30/EU) |
| ● | RoHS 2 (2011/65/EU) (we are actively working on RoHS 3 and REACH) |
| ● | Safety Requirements For Electrical Equipment For Measurement, Control, And Laboratory Use - Part 1: |
| ● | General Requirements [UL 61010-1:2012 Ed.3+R:29Apr2016] |
| ● | Safety Requirements For Electrical Equipment For Measurement, Control, And Laboratory Use – Part 1: |
| ● | General Requirements (R2017) [CSA C22.2#61010-1-12:2012 Ed.3+U1;U2] |
| ● | IEC 60601-1 - Medical electrical equipment - Part 1: General requirements for basic safety and essential performance |
| ● | IEC 61326-1:2012 - Electrical Equipment For Measurement, Control And Laboratory Use - EMC Requirements - Part 1: General Requirements |
| ● | ASME SEC. VIII Pressure Vessel Code (Fusion Only) |
| ● | EU Pressure Equipment Directive (EU97/23/EC) (Fusion Only) |
| ● | FCC 47 CFR Class B Verification (Fusion Only) |
| ● | IEC 62304 Medical device software — Software life cycle processes |
23
These standards are woven into our development methodology used to design all new products within the organization. This development process includes a risk management assessment done in accordance with ISO 14971 that identifies hazards and mitigates risks via design improvements, process improvement, and warnings (including labels and safety information shipped with the product).
We pride ourselves on our exceptional operational quality. Our temperature-controlled supply chain solutions focused on cell and gene therapies boast a 99.89% delivery success rate and due to this performance 13,477 additional patients were able to receive therapies over the past 24 months and 1,334 intended parents are potentially able to have successful cycles resulting in the birth of a child on an annual basis because of our CryoStork® solution.
While rare, recalls of product may become necessary. The primary responsibility for recall management lies with our Vice President of Quality Assurance and Regulatory Affairs for manufacturing. The executive staff is involved in decision and implementation processes depending upon the specifics of any recall required. Customer service personnel, sales staff and other resources would then be utilized in reaching all distributors and direct end users. Results of recalls are evaluated daily until the recall is closed. There were no product recalls during 2021.
Product Lifecycle Management
Cryoport creates unique products with long-term use in mind. Cryoport products are primarily constructed of recyclable aluminum or stainless steel and we approach the extension of product lifecycles through the following four areas:
| ● | Longevity |
| ● | Repairability |
| ● | Reusability |
| ● | Recyclability |
We strive for a product base with long use phases to minimize impact associated with production of new product. At our MVE Biological Solutions production facility, we manufacture fusion freezer units that utilize 1/587 of the energy used by conventional mechanical freezers used for similar applications. For example, our freezer production displaced annual electricity consumption by 139,424,817 kWh from what would otherwise be consumed from alternative products. This amount of electricity could power 11,899 homes (sized at 2,500 square feet) annually. This reduction in energy consumption from our freezer lines alone equates to 123,444,781 pounds of GHG emissions avoided or the emissions equivalent to 21,489 passenger vehicles driven for one year.
Cryoport regularly reviews opportunities to eliminate the use of materials considered hazardous and related managed waste streams on a regular cadence. Cryoport does not utilize any substances of concern in our products; We do currently utilize minimal quantities of hazardous materials that are not listed substances of concern in our operations, primarily in the form of isopropanol, epoxies, butyl cellosolve, lacquer thinner, paint, hyamine and isopropyl alcohol. These materials and the insignificant quantities of hazardous wastes generated in our production facilities are managed in compliance with all state and federal regulations. Any hazardous waste that is generated is tracked and managed with an overall goal of eliminating hazardous materials where possible.
Cryoport does not utilize any minerals identified as conflict minerals (tin, tungsten, tantalum and gold) and makes our best effort to ensure that our suppliers practice responsible sourcing practices, as further detailed in Supplier Management under our Governing Ethically pillar.
Governing Ethically
Cryoport recognizes constructive supplier relationships as essential to our ability to meet customer requirements for quality solutions. We expect our business partners to share our commitment to ethics, integrity, compliance, safety, human rights, data security, and environmental protection. By the same token, as a provider accountable to thousands of companies worldwide, we pledge, through our ESG performance, to meet or exceed our clients’ requirements for the same.
Business Ethics
We are committed to operating with honesty, truthfulness and transparency in accordance to the highest ethical and corporate governance standards – mutual respect, integrity and trust are our foundation. As an ethical operator, we have developed a robust Code of Conduct and hold ourselves accountable to it in all we do. All employees across our operations are provided with training and
24
reference materials to reinforce this commitment to integrity and ethics in our business. Our policies are clearly defined, published in local languages where applicable, and include guidance on topics including, but not limited to:
| ● | Corruption |
| ● | Anti-Trust and Anti-Competitive Behavior |
| ● | Insider Dealings |
| ● | Gifts |
| ● | Bribes (e.g., explicit prohibition of facilitation payments) |
| ● | Conflicts of Interest |
| ● | Intellectual Property |
| ● | Compliance |
| ● | Truthful and accurate reporting |
| ● | Interactions with Healthcare professionals |
| ● | Whistleblower protections (including non-retaliation) |
| ● | Political Activity and Contributions (e.g., explicit prohibition of contribution of any kind to any candidate or political party without express prior approval of the Board of Directors – this covers both direct contributions and indirection support; no political contributions have been made in recent years) |
In addition to our Code of Conduct, our senior leadership team actively oversees the governance of our ethics programs to help ensure that commitment is driven from the top down, and that program owners are accountable for successful program compliance.
Cryoport does not conduct clinical trials, animal testing or use human tissue of any kind in the manufacture or design of our products, and our Code of Conduct governs the ethical behavior of our employees across Cryoport operations. Further, the Company does not conduct lobbying activities.
Supplier Management
Temperature controlled supply chain support to the life sciences industry is critical to all that Cryoport does; therefore, we take an active approach to managing suppliers and partners to ensure that appropriate compliance, health, safety, labor practices, and ethical standards are employed. Our internal diligence process for third-party vendors including a supplier questionnaire that is required for vendor approval and a regular auditing scheme thereafter for existing suppliers. The questionnaire is intended to verify that programs exist to manage material risk areas associated with the given supplier’s operations and particular consideration is paid to diligencing bribery or other forms of corrupt activity. No suppliers are approved until this mandatory due diligence is complete and a completed assessment form is on file.
As an example of verification that programs exist to manage material risks for any given supplier, if our transportation suppliers employ or work with a Dangerous Goods Safety Advisor, we confirm the presence of a credentialed role responsible for overseeing activities associated with dangerous goods, including but not limited to, employee training and coaching, reporting, and monitoring of activities associated with the transportation of dangerous goods. The purpose of this inquiry is to gauge the degree of oversight over dangerous goods management by our suppliers to help ensure product and employee welfare.
Our Code of Conduct extends through our suppliers and thus sets an expectation for our suppliers to commit to operating with honesty, truthfulness and transparency in accordance to the highest ethical and corporate governance standards, as Cryoport personifies through our operations. Per our Code of Conduct, Cryoport will not tolerate the use by suppliers of forced labor in any form.
Data Privacy & Security
Cryoport uses an outside Center for Internet Security (CIS) assessment firm to evaluate its data security controls in an effort protect our businesses and secure the information of our employees and customers. The evaluation process utilizes the CIS Critical Security Controls Capability Maturity Model Integration (CMMI) methodology, and is an ongoing initiative used to continuously improve the CMMI rating for the Company.
25
Cryoport Impact Statements
Examples of some of our positive environmental impacts for 2020 and 2021 include the following:
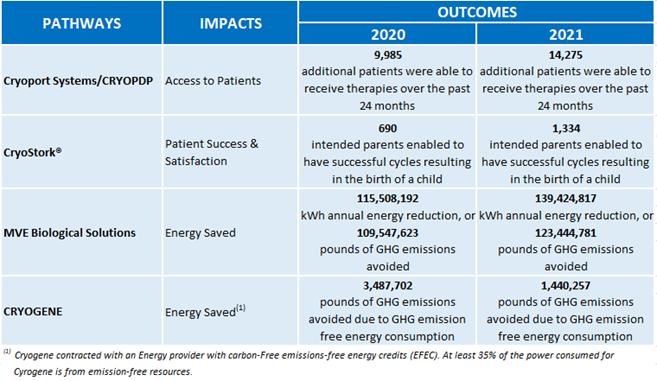
Impact of COVID-19
Refer to the sections entitled “Risks Factors” for risks we face due to the COVID-19 pandemic and “Management’s Discussion and Analysis of Financial Condition and Results of Operations — Impact of COVID-19” for information on the impact of COVID-19 on the Company.
Employees
We refer to our employees as our “team.” They are critical to our success, and we are in constant communication and training. We believe that we have assembled a strong management and leadership team with the experience and expertise needed to execute our business strategy. As of December 31, 2021, we had 847 employees: 791 full-time, 15 part-time, and 41 temporary, of which 411 are located in the Americas, 184 in EMEA and 252 in APAC. This is an increase of over 220 employees since December 31, 2020, primarily as a result of the further build out of our global organization to support the expected growth in the markets we serve. We anticipate hiring additional personnel as required to implement our global growth strategy.
Corporate History and Structure
We are a Nevada corporation originally incorporated under the name G.T.5-Limited (“GT5”) on May 25, 1990. In connection with a Share Exchange Agreement in March 2005, we changed our name to Cryoport, Inc. and acquired all of the issued and outstanding shares of common stock of Cryoport Systems, Inc., a California corporation. Cryoport Systems, Inc., which was originally formed in 1999 as a California limited liability company, was reorganized into a California corporation on December 11, 2000 and converted into Cryoport Systems, LLC, a California limited liability company, on September 17, 2020, remains one of our operating companies under Cryoport, Inc. Our principal executive offices are located at 112 Westwood Place, Suite 350, Brentwood, TN 37027. The telephone number of our principal executive office is (949) 470-2300, and our main corporate website is www.cryoport.com. The information on or that can be accessed through our website is not part of this Form 10-K.
26
Information about our Executive Officers
The following are our executive officers as of the filing date of this Form 10-K:
Jerrell W. Shelton. Mr. Shelton became a member of our board of directors in October 2012 and was appointed President and Chief Executive Officer of the Company in November 2012. He was appointed Chairman of the Board in October 2015. He served on the Board of Directors and standing committees of Solera Holdings, Inc. from April 2007 through November 2011. From June 2004 to May 2006, Mr. Shelton was the Chairman and CEO of Wellness, Inc., a provider of advanced, integrated hospital and clinical environments. Prior to that, he served as Visiting Executive to IBM Research and Head of IBM’s WebFountain. From October 1998 to October 1999, Mr. Shelton was Chairman, President and CEO of NDC Holdings II, Inc. Between October 1996 and July 1998, he was President and CEO of Continental Graphics Holdings, Inc. From October 1991 to July 1996, Mr. Shelton served as President and CEO of Thomson Business Information Group. Mr. Shelton has a B.S. in Business Administration from the University of Tennessee and an M.B.A. from Harvard University. Mr. Shelton’s extensive leadership, management, strategic planning and financial expertise through his various leadership and directorship roles in public, private and global companies, makes him well-qualified to serve as a member of the board of directors.
Robert S. Stefanovich. Mr. Stefanovich became Chief Financial Officer and Treasurer for the Company in June 2011. In 2019, he was also given the title Senior Vice President. From 2011 to 2019, Mr. Stefanovich served as the Secretary of the Company. From June 15, 2012 to November 4, 2012, Mr. Stefanovich served as the Principal Executive Officer of the Company. From November 2007 through March 2011, Mr. Stefanovich served as Chief Financial Officer of Novalar Pharmaceuticals, Inc., a venture-backed specialty pharmaceutical company. Prior to that, he held several senior positions, including interim Chief Financial Officer of Xcorporeal, Inc., a publicly traded medical device company, Executive Vice President and Chief Financial Officer of Artemis International Solutions Corporation, a publicly traded software company, Chief Financial Officer and Secretary of Aethlon Medical Inc., a publicly traded medical device company and Vice President of Administration at SAIC, a Fortune 500 company. Mr. Stefanovich also served as a member of the Software Advisory Group and an Audit Manager with Price Waterhouse LLP’s (now PricewaterhouseCoopers) hi-tech practice in San Jose, California and Frankfurt, Germany. He received his Master of Business Administration and Engineering from University of Darmstadt, Germany.
Mark Sawicki, Ph.D. Dr. Sawicki became President and Chief Executive Officer of Cryoport Systems, LLC, a wholly-owned subsidiary of the Company, and the Senior Vice President and Chief Scientific Officer of the Company in September 2020 and served as the Chief Commercial Officer of Cryoport Systems from January 2015 to August 2020. Dr. Sawicki brings over 20 years of business development and sales management experience, having consistently delivered on corporate revenue and market share goals in the pharmaceutical and biotechnology industries. Dr. Sawicki previously served as the Chief Business Officer at AAIPharma Services Corporation/Cambridge Major Laboratories Inc. (now Alcami Corporation), a contract development, testing, and manufacturing organization for pharma and biotech companies. Additionally, he has served in senior business development roles at CMC Biologics, a provider of biopharmaceutical contract manufacturing services, and Albany Molecular Research Inc. (AMRI), a contract research and manufacturing organization. Dr. Sawicki holds a bachelor's in biochemistry from the State University of New York at Buffalo and a Ph.D. in biochemistry from the State University of New York at Buffalo, School of Medicine and Biomedical Sciences. He also received graduate training at the Hauptman Woodard Medical Research Institute. Dr. Sawicki has authored a dozen scientific publications in drug discovery with a focus on oncology and immunology.
Available Information
Our main corporate website address is www.cryoport.com. The information on or that can be accessed through our website is not part of this Form 10-K. We electronically file with the SEC our Annual Report on Form 10‑K, Quarterly Reports on Form 10‑Q, Current Reports on Form 8‑K and amendments to the reports filed or furnished pursuant to Section 13(a) or 15(d) of the Exchange Act. We make available free of charge on or through our website copies of these reports as soon as reasonably practicable after we electronically file these reports with, or furnish them to, the SEC. The SEC also maintains an internet site that contains reports, proxy and information statements and other information regarding issuers that file electronically with the SEC at www.sec.gov.
ITEM 1A. RISK FACTORS
The following risk factors could materially and adversely affect our business, financial condition and results of operations. These risk factors do not identify all of the risks that we face. Our business, financial condition and results of operations could also be affected by factors that are not presently known to us or that we currently consider to be immaterial.
27
Risks Related to Our Business
We depend on the availability of certain component products used in our solutions; delays or increased costs in the procurement of components manufactured by third parties could adversely affect our business operations, financial performance and results of operations, and we may experience customer dissatisfaction and harm to our reputation.
If we fail to procure sufficient components used in our products from our third-party manufacturers, we may be unable to deliver our solutions to our customers on a timely basis, which could lead to customer dissatisfaction and could harm our reputation and ability to compete. We currently acquire various component parts for our solutions from various independent manufacturers, some of which are sole sourced. We would likely experience significant delays or cessation in producing some of these components if a labor strike, natural disaster, public health crisis or other supply disruption were to occur, including as a result of the COVID-19 pandemic, at any of our main suppliers.
For example, there is currently a worldwide shortage of semiconductor, memory and other electronic components affecting many industries. Certain of our MVE Biological Solutions products and our SmartPak system are dependent on some of these electronic components. A continued shortage of electronic components may impact us significantly and could cause us to experience extended lead times and increased prices from our suppliers. Extended lead times and decreased availability of key components could result in a significant disruption to our production schedule and our services, all of which would have an adverse effect on our business operations, financial performance and results of operations. We do not have any guarantees of supply from our third-party suppliers, and in certain cases we have limited contractual arrangements or are relying on standard purchase orders or on component parts available on the open market, which may further result in increased costs combined with reduced availability.
If we are unable to procure a component from one of our manufacturers, we may be required to enter into arrangements with one or more alternative manufacturing companies, which may cause delays in producing components or result in a significant increase in costs. Even though, we have experienced materials and transportation challenges, to date, we have not experienced any material delay that has adversely impacted our operations, but this does not mean that we will continue to have timely access to adequate supplies of essential materials and components in the future or that supplies of these materials and components will be available on satisfactory terms when needed. If our vendors for these materials and components are unable to meet our requirements, fail to make shipments in a timely manner, or ship defective materials or components, we could experience a shortage or delay in supply or fail to meet our contractual requirements, which would adversely affect our results of operations and negatively impact our cash flow and profitability. Continued delay in our ability to produce and deliver our products and services could also cause our customers to purchase alternative products and services from our competitors and/or harm our reputation.
The retirement of older telecommunications technology, such as 2G and 3G, by cellular network providers will require updates to our equipment, which could adversely impact our business operations, financial performance and results of operations.
Most providers of cellular networks globally have begun to retire their 2G cellular networks and/or have announced their intentions to retire their 2G and 3G cellular networks sometime in 2022. Certain elements of our operating model, including our SmartPak II® condition monitoring system, currently rely in part on 2G and 3G cellular networks to transmit data to our Cryoportal® Logistics Management Platform. We are currently in the process of upgrading our existing equipment that uses these cellular networks in order to meet new network standards. Additionally, current delays and uncertainties in the supply chain for semiconductor chips may impact the timely delivery to us of the sensors required to make some of these upgrades. If we are unable to upgrade our existing equipment prior to the retirement of 2G and 3G cellular networks in a timely and cost-effective manner, our business operations, financial performance and results of operations could be materially adversely affected, including due to disruptions in our ability to provide our full monitoring solutions.
Our products and services may contain errors or defects, which could result in damage to our reputation, lost revenues, diverted development resources and increased service costs, litigation and product recalls.
Our products and services must meet stringent requirements and we must develop our products and services solutions quickly to keep pace with the rapidly changing market. Product and services as sophisticated as ours could contain undetected errors or defects, especially when first introduced or when new equipment or versions of our software are released. If our product and services are not free from errors or defects, we may incur an injury to our reputation, lost revenues, diverted development resources, increased customer service and support costs, product recalls and litigation. The costs incurred in correcting any product errors or defects may be substantial and could adversely affect our business, results of operations and financial condition.
28
Due to the low temperatures at which some of our products are used and the fact that some of our products are relied upon by our customers or end users in their facilities or operations or are manufactured for relatively broad medical, transportation, or consumer use, we face an inherent risk of exposure to claims in the event that the failure, use, or misuse of our products results, or is alleged to result, in death, bodily injury, property or sample damage, or economic loss. In addition, we specialize in the secure storage of biological specimens, materials and samples covering the full range of temperatures from cryogenic through controlled room temperature. Any damage to these specimens, materials and samples may be attributed to a failure of our storage systems or services, which could lead to claims for damages made by customers and could also harm our relationship with customers and damage our reputation in the life sciences industry, resulting in material harm to our business.
Although we currently maintain product liability coverage, which we believe is adequate for product liability claims and for the continued operation of our business, it includes customary exclusions and conditions, may not cover certain specialized applications and generally does not cover warranty claims. Additionally, such insurance may become difficult to obtain or be unobtainable in the future on terms acceptable to us. A successful product liability claim or series of claims against us, including one or more consumer claims purporting to constitute class actions or claims resulting from extraordinary loss events, in excess of or outside our insurance coverage, or a significant warranty claim or series of claims against us, could materially decrease our liquidity, impair our financial condition, and adversely affect our results of operations. See “—Risks Related to Our Business—Our products and services may expose us to liability in excess of our current insurance coverage” for additional information.
In addition, regardless of merit or eventual outcome, product liability claims may result in, among other things, costs of litigation, distraction of management’s attention from our primary business, the inability to commercialize our existing or new products, decreased demand for our products or, if cleared or approved, products in development, damage to our business reputation, product recalls or withdrawals from the market, withdrawal of clinical trial participants, substantial monetary awards to patients or other claimants, or loss of revenue.
While we may attempt to manage our product liability exposure by proactively recalling or withdrawing from the market any defective products, any recall or market withdrawal of our products may delay the supply of those products to our customers and may impact our reputation. We can provide no assurance that we will be successful in initiating appropriate market recall or market withdrawal efforts that may be required in the future or that these efforts will have the intended effect of preventing product malfunctions and the accompanying product liability that may result. Additionally, any recall could result in significant costs to us and significant adverse publicity, which could harm our ability to market our products in the future. Such recalls and withdrawals may also be used by our competitors to harm our reputation for safety or be perceived by patients as a safety risk when considering the use of our products. Though it may not be possible to quantify the economic impact of a recall, it could have a material adverse effect on our business, financial condition and results of operations.
Additionally, for some of our products we offer a limited warranty for product returns which are due to defects in quality and workmanship. We attempt to estimate our potential liability for future product returns and establish reserves on our financial statements in amounts that we believe will be sufficient to address our warranty obligations; however, our actual liability for product returns may significantly exceed the amount of our reserves. If we underestimate our potential liability for future product returns, or if unanticipated events result in returns that exceed our historical experience, our financial condition and operating results could be materially and adversely affected.
The global pandemic caused by COVID-19 has already and may continue to adversely affect our business operations, financial performance and results of operations, the extent of which is uncertain and difficult to predict.
In late 2019, a novel strain of coronavirus (“COVID-19”) was reported to have surfaced in Wuhan, China, which has since spread globally. In March 2020, the World Health Organization declared COVID-19 a global pandemic. The pandemic has negatively impacted the global economy, disrupted global supply chains and reduced workforce participation. Further, the COVID-19 outbreak has resulted in government authorities around the world implementing numerous measures to try to reduce the impact of COVID-19, such as travel bans and restrictions, quarantines, shelter in place or total lock-down orders. Many countries around the world have also implemented the temporary closure of non-essential businesses and other material limitations on the conduct of business.
29
More recently, new variants of COVID-19, such as the Delta and Omicron variants, that are significantly more contagious than previous strains, have emerged. The spread of these new strains are causing many government authorities and businesses to reimplement prior restrictions, or impose new restrictions, in an effort to lessen the spread of COVID-19 and its variants. In addition, virus containment efforts as a result of governmental actions or policies or other initiatives have led to the disruption in the global supply chain and as a result, we have experienced difficulties sourcing materials and equipment and may incur additional direct costs to provide our solutions in the future. As a result of the COVID-19 outbreak and the related responses from government authorities, our business operations, financial performance and results of operations were adversely affected in 2020 due to reduced demand for our services in all markets. See “Management’s Discussion and Analysis of Financial Condition and Results of Operations–Recent Developments – Impact of COVID-19” for additional information.
Additionally, our business operations, financial performance and results of operations have been and could be further adversely impacted in a number of ways, including, but not limited to, the following:
| ● | disruptions to our operations, including a shutdown of one or more of our global logistics centers or our biostorage facility; restrictions on our operations and sales, marketing and distribution efforts; and interruptions to our research and development activities, engineering, design and manufacturing processes and other important business activities; |
| ● | further reduced demand for our products and services due to disruptions to the businesses and operations of our customers, which may, in particular, result from lower volumes of clinical studies and trials or reduced activity in the human reproductive medicine markets due to social distancing restrictions; and reduction in our animal health market due to reduced demand; |
| ● | interruptions, unavailability or delays in global shipping to transport our products; |
| ● | further disruptions, slowdowns or stoppages in the supply chain for our products; |
| ● | our ability to set up global supply chain centers in certain geographic regions; |
| ● | limitations on employee resources and availability, including due to sickness, government restrictions, labor supply shortages and the desire of employees to avoid contact with large groups of people or mass transit disruptions; |
| ● | a continuation or worsening of general economic conditions, including increased inflation; |
| ● | a fluctuation in foreign currency exchange rates or interest rates could result from market uncertainties; |
| ● | an increase in the cost or the difficulty to obtain debt or equity financing could affect our financial condition or our ability to fund operations or future investment opportunities; and |
| ● | an increase in regulatory restrictions or continued market volatility could hinder our ability to execute strategic business activities, including acquisitions, as well as negatively impact our stock price. |
The spread of COVID-19 has caused us to modify our business practices (including employee travel, employee work locations, and cancellation of physical participation in meetings, events and conferences), and we may take further actions as may be required by government authorities or that we determine are in the best interests of our employees, customers, partners, and suppliers. There is no certainty that such measures will be sufficient to mitigate the risks posed by the virus, and our ability to perform critical functions could be harmed.
Additionally, COVID-19 could negatively affect our internal controls over financial reporting as a portion of our workforce is required to work from home and therefore new processes, procedures, and controls could be required to respond to continuing changes in our business environment. Further, should any key employees become ill from COVID-19 and unable to work, the attention of the management team could be diverted.
The potential effects of COVID-19 may also impact and potentially heighten many of our other risk factors discussed in this “Risk Factors” section. The degree to which COVID-19 impacts our business operations, financial performance and results of operations will depend on future developments, which are highly uncertain, continuously evolving and cannot be predicted, including, but not limited to, the duration and spread of the COVID-19 outbreak and its variants; its severity; the actions to contain the virus or treat its impact, such as the availability and efficacy of vaccines (particularly with respect to emerging strains of the virus), and the potential hesitancy to utilize them; and how quickly and to what extent normal economic and operating conditions can resume.
30
As an increasingly global business, we are exposed to economic, political, and other risks in different countries which could materially reduce our sales, profitability or cash flows, or materially increase our liabilities.
Since we manufacture and sell our products worldwide, our business is subject to risks associated with doing business internationally. Our future results could be harmed by a variety of factors, including:
| ● | changes in foreign currency exchange rates, exchange controls and currency restrictions; |
| ● | changes in a specific country’s or region’s political, social or economic conditions; |
| ● | civil unrest, turmoil or outbreak of disease or illness, such as COVID-19, in any of the countries in which we sell our products or in which we or our suppliers operate; |
| ● | tariffs, other trade protection measures, and import or export licensing requirements; |
| ● | potentially negative consequences from changes in U.S. and international tax laws; |
| ● | difficulty in staffing and managing geographically widespread operations; |
| ● | requirements relating to withholding taxes on remittances and other payments by subsidiaries; |
| ● | restrictions on our ability to own or operate subsidiaries, make investments or acquire new businesses in these jurisdictions; |
| ● | restrictions on our ability to repatriate dividends from our foreign subsidiaries; |
| ● | difficulty in collecting international accounts receivable; |
| ● | difficulty in enforcement of contractual obligations under non-U.S. law; |
| ● | transportation delays or interruptions; and |
| ● | changes in regulatory requirements including as it relates to protection of our intellectual property. |
We will have difficulty increasing our revenues if we experience delays, difficulties or unanticipated costs in establishing the sales, marketing and distribution capabilities necessary to successfully commercialize our solutions.
We plan to further enhance our sales, marketing and distribution capabilities in the Americas, EMEA, and APAC. It will be expensive and time-consuming for us to develop and integrate our global marketing and sales network and thus we intend to further broaden our strategic alliances with domestic and international providers of shipping services and other solutions providers to the life sciences industry to incorporate use of our platform of solutions in their service offerings. We may not be able to provide adequate incentive to our sales force or to establish and maintain favorable distribution and marketing collaborations with others to promote our solutions. In addition, any third party with whom we have established a marketing and distribution relationship may not devote sufficient time to the marketing and sales of our solutions, thereby exposing us to potential expenses in exiting such distribution agreements. We, and any of our alliance partners, must also market our services in compliance with federal, state, local and international laws relating to the provision of incentives and inducements. Violation of these laws can result in substantial penalties. Therefore, if we are unable to successfully motivate and expand our marketing and sales force and further develop our sales and marketing capabilities, or if our alliance partners fail to promote our solutions, we will have difficulty increasing our revenues and the revenue may not offset the additional expense of expansion.
We expect to base our equipment and inventory purchasing decisions on our forecasts of customers’ demand, and if our forecasts are inaccurate, our operating results could be materially harmed.
As our customer base increases, we expect the need to purchase additional equipment and inventory. Our forecasts will be based on multiple assumptions, each of which may cause our estimates to be inaccurate, affecting our ability to provide products to our customers. When demand for our products increases significantly, we may not be able to meet demand on a timely basis, and we may need to expend a significant amount of time working with our customers to allocate limited supply and maintain positive customer relations, or we may incur additional costs in order to rush the manufacture and delivery of additional products. If we underestimate customers’ demand, we may forego revenue opportunities, lose market share and damage our customer relationships. Conversely, if we overestimate customer demand, we may purchase more equipment and inventory than we are able to use or sell at any given time or at all. As a result of our failure to properly estimate demand for our products, we could have excess or obsolete equipment and/or inventory, resulting in a decline in the value of our equipment and/or inventory, which would increase our costs of revenues and reduce our liquidity. Our failure to accurately manage our equipment purchases and inventory relative to demand would adversely affect our operating results.
31
If we suffer a disruption or loss to our factories, facilities or distribution system due to factors outside of our control, our operations could be seriously harmed.
We rely on our distribution system including third-party shipment and carrier services to transport our shippers containing biological material. These third-party operations could be subject to natural disasters, adverse weather conditions, other business disruptions, and carrier error, which could cause delays in the delivery of our shippers, which in turn could cause serious harm to the biological material being shipped. As a result, any prolonged delay in shipment, whether due to technical difficulties, power failures, break-ins, destruction or damage to carrier facilities as a result of a natural disaster, fire, or any other reason, including as a result of the COVID-19 pandemic, could result in damage to the contents of the shipper.
Additionally, our factories and facilities may be subject to catastrophic loss due to fire, flood, terrorism, increasing severity or frequency of extreme weather events, or other natural or man-made disasters, as well as disruptions due to a widespread outbreak of an illness or any other public health crisis, such as the COVID-19 pandemic. For example, certain components of our key products are manufactured in China, and the extent to which our ability to produce such products is affected by COVID-19 will largely continue to depend on future developments, which are highly uncertain and cannot be accurately predicted. See “-Risks Related to Our Business-The global pandemic caused by COVID-19 has already and may continue to adversely affect our business operations, financial performance and results of operations, the extent of which is uncertain and difficult to predict” for additional information. Additionally, we operate facilities that specialize in the secure storage of biological specimens, materials and samples. If natural disasters or similar events, like hurricanes, fires or explosions or large-scale accidents or power outages, were to occur that prevented us from using all or a significant portion of these facilities, damaged critical infrastructure or our customers’ biological samples, or otherwise disrupted operations at such facilities , this could affect our ability to maintain ongoing operations and cause us to incur significant expenses. Insurance coverage may not be adequate to fully cover losses in any particular case.
Our products and services may expose us to liability in excess of our current insurance coverage.
Our platform of products and services involve significant risks of liability, which may substantially exceed the revenues we derive from them. We cannot predict the magnitude of these potential liabilities. We currently maintain general liability insurance and product liability insurance. Claims may be made against us that exceed the limits of these policies.
Our liability policy is an “occurrence” based policy. Thus, our policy is complete when we purchased it and following cancellation of the policy it continues to provide coverage for future claims based on conduct that took place during the policy term. Our insurance coverage, however, may not protect us against all liability because our policies typically have various exceptions to the claims covered and also require us to assume some costs of the claim even though a portion of the claim may be covered. In addition, if we expand into new markets, we may not be aware of the need for, or be able to obtain insurance coverage for such activities or, if insurance is obtained, the dollar amount of any liabilities incurred could exceed our insurance coverage. A partially or completely uninsured claim, if successful and of significant magnitude, could have a material adverse effect on our business, financial condition and results of operations.
If we use biological and hazardous materials in a manner that causes injury, we could be liable for damages.
Our customers may ship potentially harmful biological materials in our dewars. We cannot eliminate the risk of accidental contamination or injury to employees or third parties from the use, storage, handling or disposal of these materials. In the event of contamination or injury, we could be held liable for any resulting damages, and any liability could exceed our resources or any applicable insurance coverage we may have. Additionally, we are subject to, on an ongoing basis, federal, state and local laws and regulations governing the use, storage, handling and disposal of these materials and specified waste products. In the event of an accident, we could be held liable for damages.
We operate in a competitive industry and if we cannot compete effectively, we will lose business.
We expect to continue to experience significant and increasing levels of competition in the future. While there are technological and marketing barriers to entry, we cannot guarantee that these barriers will be sufficient to defend our market share against current and future competitors. Our principal competitive considerations in our market include:
| ● | financial resources to allocate to proper marketing and an appropriate sales effort; |
| ● | acceptance of our solutions model; |
32
| ● | acceptance of our solutions including per use fee structures and other charges for services; |
| ● | keeping up technologically with ongoing development of enhanced features and benefits; |
| ● | the ability to develop and maintain and expand strategic alliances; |
| ● | establishing our brand name; |
| ● | our ability to deliver our solutions to our customers when requested; and |
| ● | our timing of introductions of new solutions and services. |
Our future revenue stream depends to a large degree on our ability to bring new solutions and services to market on a timely basis. We generally sell our products in industries that are characterized by increased competition through frequent innovation, rapid technological changes and changing industry standards. Without the timely introduction of new products, services and enhancements, our products and services may become obsolete over time, in which case our revenue and operating results could suffer.
There may also be other companies which are currently developing competitive products and services or which may in the future develop technologies and products that are comparable, superior or less costly than our own. For example, some specialty couriers and packaging manufacturers with greater resources currently provide temperature-controlled packaging solutions and may develop other products or solutions in the future, both of which compete with our products. A competitor that has greater resources than us may be able to develop and expand their networks and product offerings more quickly, devote greater resources to the marketing and sale of their solutions and adopt more aggressive pricing policies. We may not be able to successfully compete with a competitor that has greater resources, which may adversely affect our business.
If we successfully develop products and/or services, but those products and/or services do not achieve and maintain market acceptance, our business will not be profitable.
The degree of acceptance of our platform of existing products and services or any future products or services by our current target markets, and any other markets to which we attempt to sell our products and services, as well as our profitability and growth, will depend on a number of factors including, among others, our shippers’ ability to perform and preserve the integrity of the materials shipped, relative convenience and ease of use of our shippers and/or Cryoportal®, reliability and effectiveness of our biostorage services, availability of alternative products or new technologies that make our solutions and services less desirable or competitive, pricing and cost effectiveness, effectiveness of our or our collaborators’ sales and marketing strategy and the adoption cycles of our targeted customers.
In addition, even if our products and services achieve market acceptance, we may not be able to maintain that market acceptance over time if new products or services are introduced that are more favorably received than our products and services, are more cost effective, or render our products obsolete. Although we are not aware of any other treatments or methods currently being developed that would directly compete with the methods we employ, there can be no assurance that future developments in technology will not make our technology non-competitive or obsolete, or significantly reduce our operating margins or the demand for our offerings, or otherwise negatively impact our ability to be profitable.
The integration and operation of acquired businesses may disrupt our business and create additional expenses, and we may not achieve the anticipated benefits of the acquisitions.
Integration of an acquired business involves numerous risks, including assimilation of operations of the acquired business and difficulties in the convergence of systems and processes, the diversion of management’s attention from other business concerns, risks of entering markets in which we have had no or only limited direct experience, assumption of unknown or unquantifiable liabilities, difficulties in completing strategic initiatives already underway in the acquired company, and unfamiliarity with partners of the acquired company, each of which could have a material adverse effect on our business, results of operations and financial condition. We cannot assure that these risks or other unforeseen factors will not offset the intended benefits of the acquisitions, in whole or in part.
Additionally, potential acquisition opportunities become available to us from time to time, and we periodically engage in discussions or negotiations relating to potential acquisitions, including acquisitions that may be material in size or scope to our business. Any acquisition may or may not occur and, if an acquisition does occur, it may not be successful in enhancing our business for one or more of the following reasons:
| ● | Any business acquired may not be integrated successfully and may not prove profitable; |
| ● | The price we pay for any business acquired may overstate the value of that business or otherwise be too high; |
33
| ● | Liabilities we take on through the acquisition may prove to be higher than we expected; |
| ● | We may fail to achieve acquisition synergies; or |
| ● | The focus on the integration of operations of acquired entities may divert management’s attention from the day-to-day operation of our businesses. |
Acquisitions and strategic investments and alliances may also require us to integrate and collaborate with a different company culture, management team, business model, business infrastructure and sales and distribution methodology, and assimilate and retain geographically dispersed, decentralized operations and personnel. Depending on the size and complexity of an acquisition, our successful integration of the entity depends on a variety of factors, including introducing new products and meeting revenue targets as expected, the retention of key employees and key customers, increased exposure to certain governmental regulations and compliance requirements and increased costs and use of resources. Further, the integration of acquired businesses is likely to result in our systems and internal controls becoming increasingly complex and more difficult to manage. Any difficulties in the assimilation of acquired businesses into our control system could harm our operating results or cause us to fail to meet our financial reporting obligations.
Even if we are able to successfully integrate acquired businesses, we may not be able to realize the revenue and other synergies and growth that we anticipated from the acquisition in the time frame that we expected, and the costs of achieving these benefits may be higher than what we expected. As a result, the acquisition and integration of acquired businesses may not contribute to our earnings as expected and we may not achieve the other anticipated strategic and financial benefits of such transactions.
Risks Associated with Our Intellectual Property
Our success depends, in part, on our ability to obtain patent protection for our solutions, preserve our trade secrets, and operate without infringing the proprietary rights of others.
Our policy is to seek to protect our proprietary position by, among other methods, filing United States patent applications related to our technology, inventions and improvements that are important to the development of our business. Our patents or patent applications may be challenged, invalidated or circumvented in the future or the rights granted may not provide a competitive advantage. We intend to vigorously protect and defend our intellectual property. Costly and time-consuming litigation brought by us may be necessary to enforce our patents and to protect our trade secrets and know-how, or to determine the enforceability, scope and validity of the proprietary rights of others.
We also rely upon trade secrets, technical know-how and continuing technological innovation to develop and maintain our competitive position. We seek to protect these trade secrets, in part, by entering into confidentiality agreements and inventions assignment and work for hire agreements in connection with employment, consulting, or advisory relationships. Despite these efforts, any of these parties may breach the agreements and disclose our proprietary information, including our trade secrets, and we may not be able to obtain adequate remedies for such breaches. Additionally, our competitors may independently develop substantially equivalent proprietary information and techniques or otherwise gain access to our proprietary technology, or we may not be able to meaningfully protect our rights in unpatented proprietary technology.
Our current and potential competitors and other third parties may have or obtain patents or additional proprietary rights that would prevent, limit or interfere with our ability to make, use or sell our solutions either in the United States or internationally. Additionally, we may face assertions of claims by holders of patents alleging that we are infringing upon their patent rights, which claims may be without merit, but may nonetheless result in our incurring substantial costs of defense.
We rely upon certain critical information systems, including our Cryoportal® software platform, for the operation of our business and the failure of any critical information system could adversely impact our reputation and future revenues and we may be required to increase our spending on data and system security.
We rely upon certain critical information systems, including our Cryoportal® software platform which is used by our customers and business partners to automate the entry of orders, prepare customs documentation and facilitate status and location monitoring of shipped orders while in transit. In addition, the provision of service to our customers and the operation of our networks and systems involve the storage and transmission of significant amounts of proprietary information and sensitive or confidential data, including personal information of customers, employees and others. Our technology infrastructure and critical information systems are subject to damage or interruption from a number of potential sources, including unauthorized intrusions, cyber-attacks, software viruses or other malware, natural disasters, power failures, employee error or malfeasances and other events. Despite our best efforts, no cybersecurity
34
or emergency recovery process is failsafe, and if our safeguards fail or our technology infrastructure or critical information systems are compromised, the safety and efficiency of our operations could be materially harmed, our reputation could suffer, and we could face additional costs, liabilities, costly legal challenges. Additionally, an actual or alleged failure to comply with applicable United States or foreign data protection regulations or other data protection standards may expose us to litigation, fines, sanctions or other penalties. We do not have cyber security insurance and we may incur significant costs in the event of a successful cyber-attack against us. The cost and operational consequences of implementing, maintaining and enhancing further data or system protection measures could increase significantly to overcome increasingly intense, complex and sophisticated global cyber threats.
Risks Related to Regulatory and Legal Matters
Complying with certain regulations that apply to shipments using our solutions can limit our activities and increase our cost of operations.
Shipments using our solutions and services are subject to various regulations in the various countries in which we operate. For example, shipments using our solutions may be required to comply with the shipping requirements promulgated by the Centers for Disease Control (“CDC”), the Occupational Safety and Health Organization (“OSHA”), the Department of Transportation (“DOT”) as well as rules established by the IATA and the ICAO. Additionally, our data logger may be subject to regulation and certification by the Food and Drug Administration (“FDA”), Federal Communications Commission (“FCC”), and the Federal Aviation Administration (“FAA”). We will need to ensure that our solutions and services comply with relevant rules and regulations to make our solutions and services marketable, and in some cases, compliance is difficult to determine. Significant changes in such regulations could require costly changes to our solutions and services or prevent use of our shippers for an extended period of time while we seek to comply with changed regulations. If we are unable to comply with any of these rules or regulations or fail to obtain any required approvals, our ability to market our solutions and services may be adversely affected. In addition, even if we are able to comply with these rules and regulations, compliance can result in increased costs. In either event, our financial results and condition may be adversely affected. We depend on our business partners and unrelated and frequently unknown third-party agents in foreign countries to act on our behalf to complete the importation process and to make delivery of our shippers to the final user. The failure of these third parties to perform their duties could result in damage to the contents of the shipper resulting in customer dissatisfaction or liability to us, even if we are not at fault.
Changes in trade policy, tariff and import/export regulations may have a material adverse effect on our business, financial condition and results of operations.
Our international operations and transactions depend upon favorable trade relations between the United States and the foreign countries in which our customers and suppliers have operations. It may be time consuming and expensive for us to adapt to any changes in U.S. or international social, political, regulatory and economic conditions or in laws and policies governing foreign trade, manufacturing, development and investment in the territories or countries where we currently sell our products or conduct our business. If such changes occur, it could adversely affect our business.
35
We, along with our customers, are subject to various international governmental regulations. Compliance with or changes in such regulations may cause us to incur significant expenses, and if we fail to maintain satisfactory compliance with certain regulations, we may be forced to recall products and cease their manufacture and distribution, and we could be subject to civil or criminal penalties.
We, along with our customers, are subject to various significant international, federal, state and local regulations, including but not limited to regulations in the areas of health and safety, packaging, product content, employment, labor and immigration, import/export controls, trade restrictions and anti-competition. In addition, as a global organization, we are subject to data privacy and security laws, regulations, and customer-imposed controls in numerous jurisdictions as a result of having access to and processing confidential, personal, sensitive and/or patient health data in the course of our business. The EU's General Data Protection Regulation (“GDPR”), which became effective in May 2018, applies to our activities related to products and services that we offer to EU customers and workers. The GDPR established new requirements regarding the handling of personal data and includes significant penalties for non-compliance (including possible fines of up to 4 percent of total company revenue). Other governmental authorities around the world have passed or are considering similar types of legislative and regulatory proposals concerning data protection. Each of these privacy, security and data protection laws and regulations could impose significant limitations and increase our cost of providing our products and services where we process end user personal data and could harm our results of operations and expose us to significant fines, penalties and other damages.
We must also comply with complex foreign and U.S. laws and regulations, such as the U.S. Foreign Corrupt Practices Act, the U.K. Bribery Act, and other local laws prohibiting corrupt payments to governmental officials, anti-competition regulations and sanctions imposed by the U.S. Office of Foreign Assets Control and other similar laws and regulations. Violations of these laws and regulations could result in fines and penalties, criminal sanctions, restrictions on our business conduct and on our ability to offer our products in one or more countries, and could also materially affect our brand, our ability to attract and retain employees, our international operations, our business and our operating results. Although we have implemented policies and procedures designed to ensure compliance with these laws and regulations, there can be no assurance that our employees, contractors, or agents will not violate our policies.
These regulations are complex, change frequently and have tended to become more stringent over time. We may be required to incur significant expenses to comply with these regulations or to remedy any violations of these regulations. Any failure by us to comply with applicable government regulations could also result in the cessation of our operations or portions of our operations, product recalls or impositions of fines and restrictions on our ability to carry on or expand our operations. In addition, because many of our products are regulated or sold into regulated industries, we must comply with additional regulations in marketing our products. Any significant change in these regulations could reduce demand for our products, force us to modify our products to comply with new regulations or increase our costs of producing these products. If demand for our products is adversely affected or our costs increase, our operating results and business would suffer.
We are subject to regulation by the FDA or certain similar foreign regulatory agencies, and failure to comply with such regulations could harm our reputation, business, financial condition and results of operations.
Certain of our operations are subject to regulation by the FDA or similar foreign regulatory agencies. In addition, we may in the future develop products that are subject to regulation as medical devices by the FDA and similar foreign regulatory agencies. For example, we are aware that China’s National Medical Products Administration has had discussions that may require certain of our products to be registered as Class II medical devices. The regulations enforced by the FDA and similar foreign regulatory agencies govern a wide variety of product-related activities, including the research, development, testing, manufacture, quality control, approval, clearance, labeling, packaging, storage, record-keeping, promotion, advertising, distribution, marketing, post-approval monitoring and reporting, pricing, and export and import of pharmaceutical products. If we or any of our customers, suppliers or distributors fail to comply with FDA and other applicable foreign regulatory requirements or are perceived to potentially have failed to comply, we may face, among other things, warning letters; adverse publicity affecting both us and our customers; investigations or notices of non-compliance, fines, injunctions, and civil penalties; import or export restrictions; partial suspensions or total shutdown of production facilities or the imposition of operating restrictions; increased difficulty in obtaining required FDA clearances or approvals or foreign equivalents; seizures or recalls of our products or those of our customers; or the inability to sell our products and services. Any such FDA or other foreign regulatory agency actions could disrupt our business and operations, lead to significant remedial costs and have a material adverse impact on our financial position and results of operations.
36
Risks Related to Our Financial Condition
Historically, we have incurred significant losses and we may incur losses in the future.
As of December 31, 2021, we had an accumulated deficit of $467.5 million. In order to achieve and sustain revenue growth in the future, we must expand our market presence and revenues from existing and new customers. We may continue to incur losses in the future and may never generate revenues sufficient to become profitable or to sustain profitability. Continuing losses may impair our ability to raise the additional capital required to continue and expand our operations.
Our indebtedness and liabilities could limit the cash flow available for our operations and expose us to risks that could adversely affect our business, financial condition and results of operations.
We have a substantial amount of indebtedness. As of December 31, 2021, we had approximately $471.1 million of indebtedness and other liabilities, including trade payables, on a consolidated basis. We may also incur additional indebtedness to meet future financing needs. Our indebtedness could have significant negative consequences for our security holders and our business, results of operations and financial condition by, among other things:
| ● | increasing our vulnerability to adverse economic and industry conditions; |
| ● | limiting our ability to obtain additional financing; |
| ● | requiring the dedication of a substantial portion of our cash flow from operations to service our indebtedness, which will reduce the amount of cash available for other purposes; |
| ● | limiting our flexibility to plan for, or react to, changes in our business; |
| ● | diluting the interests of our existing stockholders as a result of issuing shares of our common stock upon conversion of any convertible indebtedness; and |
| ● | placing us at a possible competitive disadvantage with competitors that are less leveraged than us or have better access to capital. |
Our business may not generate sufficient funds, and we may otherwise be unable to maintain sufficient cash reserves, to pay amounts due under our indebtedness, including our outstanding convertible senior notes (collectively, the “Convertible Senior Notes”) consisting of our 3.00% convertible senior notes due 2025 (the “2025 Convertible Senior Notes”) and our 0.75% convertible senior notes due 2026 (the “2026 Convertible Senior Notes”), and our cash needs may increase in the future. In addition, any future indebtedness that we may incur may contain financial and other restrictive covenants that limit our ability to operate our business, raise capital or make payments under our other indebtedness. If we fail to comply with these covenants or to make payments under our indebtedness when due, then we would be in default under that indebtedness, which could, in turn, result in that and our other indebtedness becoming immediately payable in full.
Risks Related to Our Preferred Stock
The issuance of shares of our Series C Preferred Stock reduces the relative voting power of holders of our common stock, dilutes the ownership of such holders, and may adversely affect the market price of our common stock.
In connection with financing our acquisition of MVE Biological Solutions, on October 1, 2020, we completed the sale of 250,000 shares of a newly designated Series C Convertible Preferred Stock, par value $0.001 (“Series C Preferred Stock”), at a price of $1,000 per share, the original purchase price, to funds affiliated with The Blackstone Group Inc., or Blackstone. The holders of our Series C Preferred Stock are entitled to dividends at a rate of 4.0% per annum, paid-in-kind, accruing daily and paid quarterly in arrears and are also entitled to participate in dividends declared or paid on the common stock on an as-converted basis.
Each holder of our Series C Preferred Stock (collectively, the “Series C Preferred Stockholders”) has the right, at its option, to convert its Series C Preferred Stock, in whole or in part, into common stock at a conversion price equal to $38.6152 per share subject to certain customary adjustments. After October 1, 2022, subject to certain conditions, we may, at our option, require conversion of all of the outstanding shares of Series C Preferred Stock to common stock if, for at least 20 trading days during the 30 consecutive trading days immediately preceding the date we notify the Series C Preferred Stockholders of the election to convert, the closing price of our Common Stock is at least 150% of the conversion price. On February 5, 2021, the Company received a waiver and conversion notice from Blackstone Freeze Parent L.P. and Blackstone Tactical Opportunities Fund – FD L.P. and converted an aggregate of 50,000 shares of the Series C Preferred Stock, resulting in the issuance of an aggregate of 1,312,860 shares of common stock.
37
Any conversion of shares of the Series C Preferred Stock to shares of our common stock would dilute the ownership interest of existing holders of our Common Stock, and any sale in the public market of shares of our common stock issuable upon conversion of the Series C Preferred Stock could adversely affect prevailing market prices of our common stock. Additionally, we granted the Series C Preferred Stockholders customary registration rights in respect of their securities. These registration rights facilitate the resale of our common stock issuable upon conversion of such securities into the public market, and any such resale would increase the number of shares of our common stock available for public trading.
The Series C Preferred Stockholders may exercise influence over us, including through their ability to designate a member of our board of directors.
The Series C Preferred Stockholders are generally entitled to vote with the holders of the shares of Common Stock on all matters submitted for a vote of holders of shares of Common Stock (voting together with the holders of shares of Common Stock as one class) on an as-converted basis, subject to certain NASDAQ voting limitations, if applicable. Additionally, the consent of the holders of a majority of the outstanding shares of Series C Preferred Stock is required for so long as any shares of the Series C Preferred Stock remain outstanding for (i) amendments to the Company’s organizational documents that have an adverse effect on the holders of Series C Preferred Stock and (ii) issuances by the Company of securities that are senior to, or equal in priority with, the Series C Preferred Stock, including any shares of the Company’s Series A Preferred Stock or Series B Preferred Stock. In addition, for so long as 75% of the Series C Preferred Stock issued in connection with the Securities Purchase Agreement remains outstanding, the consent of the holders of a majority of the outstanding shares of Series C Preferred Stock will be required for (i) any voluntary dissolution, liquidation, bankruptcy, winding up or deregistration or delisting and (ii) incurrence by Cryoport of any indebtedness unless our ratio of debt to LTM EBITDA (as defined in the Certificate of Designation of the Series C Preferred Stock) would be less than a ratio of 5-to-1 on a pro forma basis giving effect to such incurrence and the use of proceeds therefrom.
Additionally, an affiliate of Blackstone has the right to nominate for election one member to our board of directors for so long as certain parties hold 66.67% of the Series C Preferred Stock issued in the Blackstone financing transaction. The director designated by Blackstone is entitled to serve on committees of our board of directors, subject to applicable law and NASDAQ rules. Notwithstanding the fact that all directors will be subject to fiduciary duties to us and to applicable law, the interests of the director designated by Blackstone may differ from the interests of our security holders as a whole or of our other directors.
As a result, the Series C Preferred Stockholders have the ability to influence the outcome of certain matters affecting our governance and capitalization. The sponsors of the Series C Preferred Stockholders are in the business of making or advising on investments in companies, including businesses that may directly or indirectly compete with certain portions of our business, and they may have interests that diverge from, or even conflict with, those of our other shareholders. They may also pursue acquisition opportunities that may be complementary to our business, and, as a result, those acquisition opportunities may not be available to us. Our obligations to the Series C Preferred Stockholders could also limit our ability to obtain additional financing or increase our borrowing costs, which could have an adverse effect on our financial condition.
Our Series C Preferred Stock has rights, preferences, and privileges that are not held by, and are preferential to, the rights of holders of our common stock, which could adversely affect our liquidity and financial condition.
The Series C Preferred Stockholders have the right under the Certificate of Designation of the Series C Preferred Stock to receive a liquidation preference entitling them to be paid an amount per share equal to the greater of (i) the original purchase price, plus all accrued and unpaid dividends and (ii) the amount that the holder would have been entitled to receive at such time if the Series C Preferred Stock were converted into common stock. In addition, the Series C Preferred Stockholders are entitled to dividends at a rate of 4.0% per annum, paid-in-kind, accruing daily and paid quarterly in arrears. The Series C Preferred Stockholders are also entitled to participate in dividends declared or paid on the common stock on an as-converted basis.
38
Risks Related to Ownership of Our Common Stock
Certain of our existing stockholders own and have the right to acquire a substantial number of shares of common stock.
As of February 18, 2022, our directors, executive officers and beneficial owners of 5% or more of our outstanding common stock beneficially owned 25,034,116 shares of common stock assuming their exercise of all outstanding Series C Preferred Stock and options that are exercisable within 60 days of February 18, 2022 or approximately 46.3 % of our outstanding common stock. As such, the concentration of beneficial ownership of our common stock may have the effect of delaying or preventing a change in control of Cryoport and may adversely affect the voting or other rights of other holders of our common stock.
Future sales of shares of our common stock may depress the price of our shares and be dilutive to our existing stockholders.
Future issuances of shares of our common stock or the availability of shares for resale in the open market may decrease the market price per share of our common stock. As of December, 2021, there were 49,616,154 shares of our common stock outstanding. Substantially all of these shares of common stock are eligible for trading in the public market. The market price of our common stock may decline if our stockholders sell a large number of shares of our common stock in the public market, or the market perceives that such sales may occur.
As of December 31, 2021, we could also issue up to an additional 7,401,790 shares of our common stock upon exercise of outstanding options and restricted stock units and 7,021,279 shares of our common stock reserved for future issuance under our stock incentive plans. In addition, we reserved 599,954 shares of our common stock issuable upon conversion of our 2025 convertible notes, 3,422,780 shares of our common stock issuable upon conversion of our 2026 convertible notes, and 5,443,505 shares of our common stock issuable upon conversion of our Series C Convertible Preferred Stock. The exercise of any options, the issuance of our common stock upon conversion of the 2025 convertible notes, the 2026 convertible notes, or the Series C Convertible Preferred Stock, or in connection with acquisitions and other issuances of our common stock could have an adverse effect on the market price of the shares of our common stock and dilute our existing stockholders.
To the extent that we raise additional funds through the sale of equity or convertible debt securities, the issuance of such securities will result in dilution to our stockholders, including the issuance of shares upon conversion of the notes being issued in the Concurrent Offering. Investors purchasing shares or other securities in the future could have rights superior to existing stockholders. The price per share at which we sell additional shares of our common stock, or securities convertible or exchangeable into common stock, in future transactions may be higher or lower than the price per share paid by investors in this placement. In addition, if the holders of our outstanding options exercise such securities, you may incur further dilution.
Our stock price has been and will likely continue to be volatile.
The market price of our common stock has been highly volatile and could fluctuate widely in price in response to various factors, many of which are beyond our control, including, but not limited to technological innovations or new solutions and services by us or our competitors, additions or departures of key personnel, sales of our common stock, our ability to execute our business plan, our operating results being below expectations, loss of any strategic relationship, industry developments, economic and other external factors and period-to-period fluctuations in our financial results.
In addition, the securities markets have from time to time experienced significant price and volume fluctuations that are unrelated to the operating performance of companies. These market fluctuations may also materially and adversely affect the market price of our common stock and warrants.
We have not paid dividends on our common stock in the past and do not expect to pay dividends in the foreseeable future. Any return on investment may be limited to the value of our common stock.
We have never paid cash dividends on our common stock and do not anticipate paying cash dividends in the foreseeable future. Any future determination to pay dividends will be at the discretion of our board of directors, subject to compliance with covenants in current and future agreements governing our indebtedness, and will depend on our results of operations, financial condition, capital requirements, contractual arrangements and other factors that our board of directors deems relevant. If we do not pay dividends, our common stock may be less valuable because a return on your investment will only occur if the price of our common stock appreciates.
39
Our Articles of Incorporation allows our Board of Directors to issue up to 2,500,000 shares of “blank check” preferred stock.
Our Articles of Incorporation allows our board of directors to issue up to 2,500,000 shares of “blank check” preferred stock, without action by our stockholders. We have designated 800,000 shares as Class A Preferred Stock, 585,000 shares as Class B Preferred Stock and 250,000 shares of Series C Preferred Stock, of which 200,000 shares of Series C Preferred Stock are issued and outstanding at February 18, 2022. Without limiting the foregoing, (i) such shares of preferred stock could have liquidation rights that are senior to the liquidation preference applicable to our common stock and Preferred Stock, (ii) such shares of preferred stock could have voting or conversion rights, which could adversely affect the voting power of the holders of our common stock and preferred stock and (iii) the ownership interest of holders of our common stock will be diluted following the issuance of any such shares of preferred stock. In addition, the issuance of such shares of blank check preferred stock could have the effect of discouraging, delaying or preventing a change of control of our Company.
Provisions in our bylaws and Nevada law might discourage, delay or prevent a change of control of our Company or changes in our management and, as a result, may depress the trading price of our common stock.
Provisions of our bylaws and Nevada law may discourage, delay or prevent a merger, acquisition or other change in control that stockholders may consider favorable, including transactions in which you might otherwise receive a premium for your shares of our common stock. The relevant bylaw provisions may also prevent or frustrate attempts by our stockholders to replace or remove our management. These provisions include advance notice requirements for stockholder proposals and nominations, and the ability of our Board of Directors to make, alter or repeal our bylaws.
In addition, Section 78.411, et seq. of the Nevada Revised Statutes prohibits a publicly-held Nevada corporation from engaging in a business combination with an interested stockholder (generally defined as a person which together with its affiliates owns, or within the last two years has owned, 10% of our voting stock) for a period of two years after the date of the transaction in which the person became an interested stockholder, unless the business combination is approved in a prescribed manner.
The existence of the foregoing provisions and other potential anti-takeover measures could limit the price that investors might be willing to pay in the future for shares of our common stock. They could also deter potential acquirers of our Company, thereby reducing the likelihood that you could receive a premium for your common stock in an acquisition.
General Risk Factors
Our ability to grow and compete in our industry will be hampered if we are unable to retain the continued service of our key professionals or to identify, hire and retain additional qualified professionals.
Our success in implementing our business strategy depends largely on the skills, experience and performance of key members of our executive management team and others in key management positions. The collective efforts of each of these persons working as a team will be critical to us as we continue to develop our technologies, tests and engineering and development and sales programs. As a result of the difficulty in locating qualified new management, the loss or incapacity of existing members of our executive management team could adversely affect our operations. If we were to lose one or more of these key employees, we could experience difficulties in finding qualified successors, competing effectively, developing our technologies and implementing our business strategy. We do not maintain "key person" insurance on any of our employees.
In addition, a critical factor to our business is our ability to attract and retain qualified professionals including key employees and consultants. We are continually at risk of losing current professionals or being unable to hire additional professionals as needed. If we are unable to attract new qualified employees, our ability to grow will be adversely affected. If we are unable to retain current employees or strategic consultants, our financial condition and ability to maintain operations may be adversely affected.
40
If equity research analysts do not publish research or reports about our business or if they issue unfavorable commentary or downgrade our common stock, the price of our common stock could decline.
The trading market for our common stock relies in part on the research and reports that equity research analysts publish about us and our business. We do not control these analysts. The price of our common stock could decline if one or more equity analyst downgrades our stock or if analysts downgrade our stock or issue other unfavorable commentary or cease publishing reports about us or our business.
ITEM 1B. Unresolved Staff Comments
Not applicable.
ITEM 2. Properties
Our principal executive office is located in Brentwood, Tennessee. We lease or own various corporate, global logistics and supply chain centers, biostorage, manufacturing, and research and development facilities at 33 sites across the Americas, EMEA and APAC regions.
The following table summarizes our principal facilities and other materially important physical properties as of December 31, 2021:
Location |
|
Ownership |
|
Use |
Brentwood, Tennessee |
Leased |
Principle Executive Office |
||
Irvine, California |
Leased |
Administrative, Global Supply Chain Center and Research and Development Center |
||
Morris Plains, New Jersey |
Leased |
Global Supply Chain Center |
||
Houston, Texas |
Leased |
Administrative, Global Supply Chain Center and Biostorage Center |
||
Hoofddorp, the Netherlands |
Leased |
Global Supply Chain Center |
||
Ball Ground, Georgia |
Leased |
Administrative, Manufacturing, and Research and Development Center |
||
New Prague, Minnesota |
Owned |
Manufacturing |
||
Chengdu, China |
Owned |
Administrative and Manufacturing |
||
Lisbon, Portugal |
Leased |
Administrative |
||
Tremblay-en-France, France |
Leased |
Administrative and Global Logistics Center |
We believe that these facilities are adequate, suitable and of sufficient capacity to support our immediate needs.
ITEM 3. Legal Proceedings
In the ordinary course of business, we are at times subject to various legal proceedings and disputes, including product liability claims. We currently are not aware of any such legal proceedings or claim that we believe will have, individually or in the aggregate, a material adverse effect on our business, operating results or cash flows. It is our practice to accrue for open claims based on our historical experience and available insurance coverage.
ITEM 4. Mine Safety Disclosures
Not applicable
41
PART II
ITEM 5. Market for Registrant’s Common Equity, Related Stockholder Matters and Issuer Purchases of Equity Securities
Common Stock
As of February 18, 2022 there were 49,694,787 shares of common stock outstanding and 158 stockholders of record. Because many shares of our common stock are held by brokers and other institutions on behalf of stockholders, we are unable to estimate the total number of stockholders represented by these stockholders of record.
Market Information
The Company’s common stock is currently listed on the NASDAQ Capital Market and is traded under the symbol “CYRX.”
Stock Performance Graph (1)
The graph below compares Cryoport’s cumulative 5-year total shareholder return on common stock with the cumulative total returns of the Russell 3000 Index and S&P 1500 Life Sciences Tools & Services Industry Index. The graph tracks the performance of a $100 investment in our common stock and in each index from December 31, 2016 to December 31, 2021 and assumes that, as to such indices, dividends were reinvested. We have never paid cash dividends on our common stock. The stock price performance on the following graph is not necessarily indicative of future stock price performance.
COMPARISON OF 5 YEAR CUMULATIVE TOTAL RETURN*
Among Cryoport, Inc., the Russell 3000 Index and the S&P 1500 Life Sciences Tools & Services Industry Index
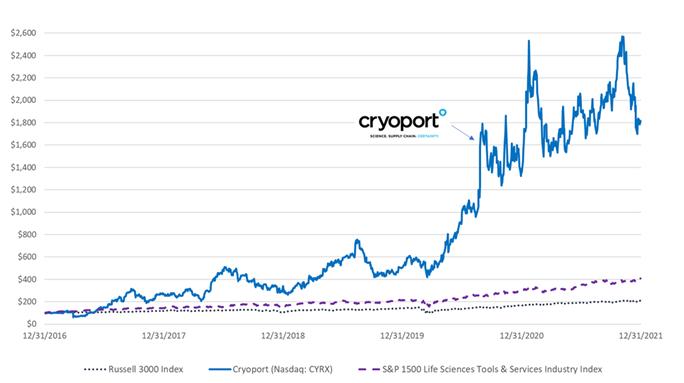
*$100 invested on 12/31/16 in Cryoport common stock or applicable index. Fiscal year ending December 31.
(1) |
The information contained in the performance graph shall not be deemed to be “soliciting material” or to be “filed” with the SEC, and such information shall not be incorporated by reference into any future filing under the Securities Act or the Exchange Act, except to the extent that Cryoport specifically incorporates it by reference into such filing. |
42
Dividends
No dividends on common stock have been declared or paid by the Company. The Company intends to employ all available funds for the development of its business and, accordingly, does not intend to pay any cash dividends in the foreseeable future. Any future determination to pay dividends will be at the discretion of our board of directors, subject to compliance with covenants in current and future agreements governing our indebtedness, and will depend on our results of operations, financial condition, capital requirements, contractual arrangements and other factors that our board of directors deems relevant.
Recent Sale of Unregistered Securities
There were no unregistered sales of equity securities during the fiscal year ended December 31, 2021 other than as reported in our Current Reports on Form 8-K filed with the SEC.
Issuer Purchases of Equity Securities
None.
ITEM 6. [Reserved]
43
ITEM 7. Management's Discussion and Analysis of Financial Condition and Results of Operations
The following discussion and analysis of our financial condition and results of our operations should be read in conjunction with our consolidated financial statements and related notes included elsewhere in this Form 10-K. Our actual results could differ materially from those contained in forward-looking statements due to a number of factors. See “Forward-Looking Statements” in this Form 10-K.
For further discussion and analysis regarding our financial condition and results of operations for the year ended December 31, 2020 as compared to the year ended December 31, 2019, refer to “Part II, Item 7 - Management's Discussion and Analysis of Financial Condition and Results of Operations” in our Annual Report on Form 10-K for the fiscal year ended December 31, 2020 filed with the SEC on March 1, 2021.
General Overview
Cryoport provides integrated temperature-controlled supply-chain solutions supporting the biopharma, animal health, and reproductive medicine markets. With over 850 employees spread across 33 locations worldwide we are focused on developing, implementing, and leveraging an industry-leading, comprehensive supply chain platform in support of the storage and distribution of critical, irreplaceable products and materials for the life sciences industry. Our supply chain solutions platform is focused on delivering unparalleled, highly differentiated temperature-controlled logistics, packaging, storage, and informatics services for products, therapies, and treatments that require unique, specialized cold chain management. Our primary focus is on addressing the critical temperature-controlled supply chain needs within the biopharmaceutical space with an emphasis on serving the rapidly growing cell and gene therapy, or C>, market.
See the “Business” section in Part I, Item 1 of this Form 10-K for additional information.
Impact of COVID-19
In late 2019, a novel strain of coronavirus that causes coronavirus disease (COVID-19) was reported to have surfaced in Wuhan, China, which has since spread globally. In March 2020, the World Health Organization declared COVID-19 a global pandemic. The pandemic has negatively impacted the global economy, disrupted global supply chains and reduced workforce participation. Further, the COVID-19 outbreak has resulted in government authorities around the world implementing numerous measures to try to reduce the impact of COVID-19, such as travel bans and restrictions, quarantines, shelter in place or total lock-down orders. Many countries around the world have also implemented the temporary closure of non-essential businesses and other material limitations on the conduct of business. As a provider of life saving therapies, Cryoport is deemed to be an essential business and has remained fully open and operational. However, the full extent and duration of this pandemic is still unknown at this point and the related governmental, business and travel restrictions in order to contain this virus are continuing to evolve globally. Accordingly, there is significant uncertainty related to the ultimate duration and impact that this global pandemic will have on future results of our operations.
Several life sciences companies, including some of our clients, announced during 2020 the temporary suspension of clinical studies and trials as well as other COVID-19 related risks that may impact their preclinical and clinical trials, including delays in patient enrollment or difficulties in initiating or expanding clinical trials, interruption of clinical trial activity, and diversion of healthcare resources to focus on COVID-19 activities. While these temporary suspensions and restrictions have been lifted such actions negatively impacted our revenue in the markets we serve during 2020. More recently, new variants of COVID-19 such as the Delta and Omicron variants, that are significantly more contagious than previous strains, have emerged. The spread of these new strains are causing many government authorities and businesses to reimplement prior restrictions, or impose new restrictions, in an effort to lessen the spread of COVID-19 and its variants. Accordingly, while we have experienced revenue ramping back up gradually over time, this may be curtailed to the extent we or our clients are affected by such restrictions in the future. Further, virus containment efforts as a result of governmental actions or policies or other initiatives have led to the disruption in the global supply chain and as a result, we have experienced difficulties sourcing materials and equipment and may incur additional direct costs to provide our solutions in the future. See “Risk Factors—Risk Related to Our Business—We depend on the availability of certain component products used in our solutions; delays or increased costs in the procurement of components manufactured by third parties could adversely affect our business operations, financial performance and results of operations, and we may experience customer dissatisfaction and harm to our reputation” in Part I, Item 1A of this Form 10-K for additional information.
44
While longer-term client demand for our services overall remains strong, the effects of the COVID-19 pandemic, including the measures taken by some of our clients , as described above, have adversely impacted our revenue growth. We continue to monitor the evolving situation caused by the COVID-19 pandemic, and we may take further actions required by governmental authorities or that we determine are prudent to support the well-being of our employees, customers, suppliers, business partners and others. The degree to which COVID-19 impacts our business operations, financial performance and results of operations will depend on future developments, which are highly uncertain, continuously evolving and cannot be predicted, including, but not limited to, the duration and spread of the COVID-19 outbreak and its variants; its severity; the actions to contain the virus or treat its impact, such as the availability and efficacy of vaccines (particularly with respect to emerging strains of the virus), and the potential hesitancy to utilize them; and how quickly and to what extent normal economic and operating conditions can resume. See “Risk Factors—Risk Related to Our Business—The recent global pandemic caused by COVID-19 has already and may continue to adversely affect our business operations, financial performance and results of operations, the extent of which is uncertain and difficult to predict” in Part I, Item 1A of this Form 10-K for additional information
Segment Reporting
Operating segments are defined as components of an enterprise about which separate financial information is available that is evaluated regularly by the chief operating decision maker in deciding how to allocate resources and in assessing operating performance. The chief operating decision maker (“CODM”) is our Chief Executive Officer. We previously reported results based on two reportable operating segments: Global Logistics Solutions and Global Bioservices. During the fourth quarter of 2020, our CODM changed how he makes operating decisions, assesses the performance of the business and allocates resources in a manner that caused our operating segments to change as a result of the MVE and CRYOPDP acquisitions. In consideration of the FASB ASC 280, Segment Reporting, we determined that we are not organized around specific products and services, geographic regions or regulatory environments. Accordingly, beginning with the fourth quarter of 2020, we operate in one reportable operating segment.
Critical Accounting Policies and Estimates
Our discussion and analysis of our consolidated financial condition and results of operations are based upon our consolidated financial statements, which have been prepared in conformity with accounting principles generally accepted in the U.S., or U.S. GAAP. While our significant accounting policies are more fully described in the notes to our consolidated financial statements, we have identified the policies and estimates below as being critical to our business operations and the understanding of our results of operations. These policies require management’s most difficult, subjective or complex judgements, often as a result of the need to make estimates about the effect of matters that are inherently uncertain. The impact of and any associated risks related to these policies on our business operations are discussed throughout “Management’s Discussion and Analysis of Financial Condition,” including in the “Results of Operations” section, where such policies affect our reported and expected financial results. Although we believe that our estimates, assumptions, and judgements are reasonable, they are based upon information presently available. Actual results may differ significantly from these estimates under different assumptions, judgments, or conditions.
The SEC defines critical accounting policies as those that are, in management’s view, most important to the portrayal of our financial condition and results of operations and most demanding of our judgment. We consider the following policies to be critical to an understanding of our consolidated financial statements and the uncertainties associated with the complex judgments made by us that could impact our results of operations, financial position and cash flows: Revenue Recognition, Business Combinations, Intangible Assets and Goodwill, Series C Preferred Stock, Stock-based Compensation, Convertible Senior Notes and Income Taxes. See Note 2: “Summary of Significant Accounting Policies” of our accompanying consolidated financial statements for a description of our critical accounting policies and estimates.
Revenue Recognition
Revenues are recognized when control is transferred to customers, in an amount that reflects the consideration the Company expects to be entitled to in exchange for those goods and services. Revenue recognition is evaluated through the following five steps: (i) identification of the contract, or contracts, with a customer; (ii) identification of the performance obligations in the contract; (iii) determination of the transaction price; (iv) allocation of the transaction price to the performance obligations in the contract; and (v) recognition of revenue when or as a performance obligation is satisfied.
45
Performance Obligations
At contract inception, an assessment of the goods and services promised in the contracts with customers is performed and a performance obligation is identified for each distinct promise to transfer to the customer a good or service (or bundle of goods or services). To identify the performance obligations, the Company considers all of the goods or services promised in the contract regardless of whether they are explicitly stated or are implied by customary business practices. Revenue is recognized when our performance obligation has been met. The Company considers control to have transferred upon delivery because the Company has a present right to payment at that time, the Company has transferred use of the asset, and the customer is able to direct the use of, and obtain substantially all of the remaining benefits from, the asset.
For arrangements under which the Company provides biological specimen storage services and logistics support and management to the customer, the Company satisfies its performance obligations as those services are performed whereby the customer simultaneously receives and consumes the benefits of such services under the agreement.
Revenue generated from short-term logistics and engineering consulting services provided to customers is recognized when the Company satisfies the contractually defined performance obligations. When a contract includes multiple performance obligations, the contract price is allocated among the performance obligations based upon the stand-alone selling prices. Approved contract modifications are accounted for as either a separate contract or as part of the existing contract depending on the nature of the modification.
Our performance obligations on our orders and under the terms of agreements with customers are generally satisfied within one year from a given reporting date and, therefore, we omit disclosure of the transaction price allocated to remaining performance obligations on open orders.
Shipping and handling activities related to contracts with customers are accounted for as costs to fulfill our promise to transfer the associated products pursuant to the accounting policy election allowed under Topic 606 and are not considered a separate performance obligation to our customers. Accordingly, the Company records amounts billed for shipping and handling as a component of revenue. Shipping and handling fees and costs are included in cost of revenues in the accompanying condensed consolidated statements of operations.
Revenues are recognized net of any taxes collected from customers, which are subsequently remitted to governmental agencies.
Business Combinations
Amounts paid for acquisitions are allocated to the tangible and intangible assets acquired and liabilities assumed, if any, based on their fair values at the dates of acquisition. This purchase price allocation process requires management to make significant estimates and assumptions with respect to intangible assets. The fair value of identifiable intangible assets is based on detailed valuations that use information and assumptions determined by management. Any excess of purchase price over the fair value of the net tangible and intangible assets acquired is allocated to goodwill. While we use our best estimates and assumptions to accurately value assets acquired and liabilities assumed at the acquisition date as well as any contingent consideration, where applicable, our estimates are inherently uncertain and subject to refinement. As a result, during the measurement period, which may be up to one year from the acquisition date, we record adjustments to the assets acquired and liabilities assumed with the corresponding offset to goodwill. Upon conclusion of the measurement period or final determination of the values of assets acquired or liabilities assumed, whichever comes first, any subsequent adjustments are recorded to our consolidated statements of operations.
We use the income approach to determine the fair value of certain identifiable intangible assets such as customer relationships. This approach determines fair value by estimating after-tax cash flows attributable to these assets over their respective useful lives and then discounting these after-tax cash flows back to a present value. We base our assumptions on estimates of future cash flows, expected growth rates, expected trends in technology, etc. We base the discount rates used to arrive at a present value as of the date of acquisition on the time value of money and certain industry-specific risk factors. We believe the estimated purchased customer relationships, agent networks, software, developed technologies, and trademarks/tradenames so determined represent the fair value at the date of acquisition and do not exceed the amount a third party would pay for the assets.
46
Intangible Assets and Goodwill
Intangible assets
Intangible assets with a definite life are amortized over their useful lives using the straight-line method, which is the best estimate of the value we are receiving over the useful life of the intangible asset and another systematic method was not deemed more appropriate. The amortization expense is recorded within selling, general and administrative expense in the consolidated statements of operations. Intangible assets and their related useful lives are reviewed at least annually to determine if any adverse conditions exist that would indicate the carrying value of these assets may not be recoverable. More frequent impairment assessments are conducted if certain conditions exist, including a change in the competitive landscape, any internal decisions to pursue new or different technology strategies, a loss of a significant customer, or a significant change in the marketplace, including changes in the prices paid for the Company’s products or changes in the size of the market for the Company’s products. If impairment indicators are present, the Company determines whether the underlying intangible asset is recoverable through estimated future undiscounted cash flows. If the asset is not found to be recoverable, it is written down to the estimated fair value of the asset based on the sum of the future discounted cash flows expected to result from the use and disposition of the asset. If the estimate of an intangible asset’s remaining useful life is changed, the remaining carrying amount of the intangible asset is amortized prospectively over the revised remaining useful life. The Company continues to believe that its definite-lived intangible assets are recoverable at December 31, 2021. There has been no impairment of our intangible assets for the periods presented.
Goodwill
We test goodwill for impairment on an annual basis in the fourth quarter or more frequently if management believes indicators of impairment exist. Events that would indicate impairment and trigger an interim impairment assessment include, but are not limited to, current economic and market conditions, including a decline in market capitalization, a significant adverse change in legal factors, business climate or operational performance of the business, and an adverse action or assessment by a regulator. Accounting guidance also permits an optional qualitative assessment for goodwill to determine whether it is more likely than not that the carrying value of a reporting unit exceeds its fair value. If, after this qualitative assessment, we determine that it is not more likely than not that the fair value of a reporting unit is less than its carrying amount, then no further quantitative testing would be necessary. A quantitative assessment is performed if the qualitative assessment results in a more likely than not determination or if a qualitative assessment is not performed. The quantitative assessment considers whether the carrying amount of a reporting unit exceeds its fair value, in which case an impairment charge is recorded to the extent the reporting unit’s carrying value exceeds its fair value. As a result of our 2021 quantitative assessment, we concluded that goodwill is not impaired as of December 31, 2021.
Series C Preferred Stock
On the October 1, 2020 issuance date, the effective conversion price per share was less than the fair value of the underlying common stock and, as a result, the Company determined that there was a beneficial conversion feature on that date. Accordingly, the Company recognized the resulting beneficial conversion feature amount of approximately $39.5 million as a deemed dividend, equal to the number of common shares into which the Series C Preferred Stock is convertible multiplied by the difference between the fair value of the common stock and the effective conversion price per share on that date. Because the Series C Preferred Stock does not have a stated conversion date and was immediately convertible at the issuance date, the dividend is reflected as a one-time, non-cash, deemed dividend to the holders of the Series C Preferred Stock on the date of issuance.
Additionally, the Company determined that the nature of the Series C Preferred Stock is more akin to an equity instrument and that the economic characteristics and risks of the embedded conversion options are clearly and closely related to the Series C Preferred Stock. As such, the conversion options were not required to be bifurcated from the host under ASC 815, Derivatives and Hedging.
Since the paid-in-kind dividends are nondiscretionary, the Company measured the beneficial conversion feature in the paid-in-kind dividend on the issuance date of the preferred stock and recorded such amount when the paid-in-kind dividend was accrued. Accordingly, the associated paid-in-kind dividends for the year ended December 31, 2020 generated a beneficial conversion feature amount of $0.3 million. On February 5, 2021, the Company received a waiver and conversion notice from Blackstone Freeze Parent L.P. and Blackstone Tactical Opportunities Fund – FD L.P. and converted an aggregate of 50,000 shares of the Series C Preferred Stock, resulting in the issuance of an aggregate of 1,312,860 shares of common stock. See Note 15: “Stockholders’ Equity” of our accompanying consolidated financial statements for additional information.
47
The Company evaluated the Series C Preferred Stock for liability or equity classification under the applicable accounting guidance including ASC 480, Distinguishing Liabilities from Equity, and determined that equity treatment was appropriate because the Series C Preferred Stock did not meet the definition of the liability instruments defined thereunder for convertible instruments. Specifically, the convertible preferred shares are not mandatorily redeemable and do not embody an obligation to buy back the shares outside of the Company’s control in a manner that could require the transfer of assets. Additionally, the Company determined that the Series C Preferred Stock would be recorded as permanent equity given that they are not redeemable for cash or other assets (i) on a fixed or determinable date, (ii) at the option of the holder, or (iii) upon the occurrence of an event that is not solely within control of the Company.
The Company also evaluated the embedded put and call options within the Series C Preferred Stock in accordance with the accounting guidance for derivatives to determine if bifurcation is required. The Company determined that the economic characteristics and risks of the embedded put and call options are not clearly and closely related to the Series C Preferred Stock. Therefore, the Company assessed the put and call options further and determined they did not meet the definition of a derivative under ASC 815.
Under the same analysis, the Company determined that the economic characteristics and risks of the embedded participating dividend feature are considered clearly and closely related to the equity host. Accordingly, the participating dividend feature is not required to be bifurcated under ASC 815. Also, the Company determined that the value of the contingent dividend feature is minimal and insignificant relative to the other components of the Series C Preferred Stock due to the circumstances surrounding the scenarios under which the provision would be triggered.
Convertible Senior Notes
The Convertible Senior Notes are accounted for in accordance with ASC 470-20, Debt with Conversion and Other Options (“ASC 470-20”) and ASC 815-40, Contracts in Entity’s Own Equity (“ASC 815-40”). Under ASC 815-40, to qualify for equity classification (or nonbifurcation, if embedded) the instrument (or embedded feature) must be both (1) indexed to the issuer’s stock and (2) meet the requirements of the equity classification guidance. Based upon the Company’s analysis, it was determined the Convertible Senior Notes do contain embedded features indexed to its own stock, but do not meet the requirements for bifurcation, and therefore do not need to be separately accounted for as an equity component. Since the embedded conversion feature meets the equity scope exception from derivative accounting, and also since the embedded conversion option does not need to be separately accounted for as an equity component under ASC 470-20, the proceeds received from the issuance of the convertible debt was recorded as a liability on the consolidated balance sheet.
Stock-based Compensation
We use the Black-Scholes option pricing model to calculate the fair value of stock option awards on the grant date. The expected option life assumption is estimated based on the simplified method. Accordingly, the Company has utilized the average of the contractual term of the options and the weighted average vesting period for all options to calculate the expected option term. The risk-free interest rate assumption is based upon observed interest rates appropriate for the expected term of our employee stock options. The expected volatility is based on the average of the historical volatility and the implied volatility of our stock commensurate with the expected life of the stock-based award. We do not anticipate paying dividends on the common stock in the foreseeable future.
We recognize stock-based compensation cost on a straight-line basis over the vesting period. Stock-based compensation expense is recognized only for those awards that ultimately vest.
48
Income Taxes
Deferred taxes are determined based on the difference between the financial statement and tax basis of assets and liabilities using enacted tax rates in effect in the years in which the differences are expected to reverse. Valuation allowances are provided, if, based upon the weight of available evidence, it is more likely than not that some or all of the deferred tax assets will not be realized. We account for uncertain tax positions using a “more-likely-than-not” threshold for recognizing and resolving uncertain tax positions. The evaluation of uncertain tax positions is based on factors including, but not limited to, changes in tax law, the measurement of tax positions taken or expected to be taken in tax returns, the effective settlement of matters subject to audit, new audit activity and changes in facts or circumstances related to a tax position. We evaluate our tax position on a quarterly basis. We also accrue for potential interest and penalties related to unrecognized tax benefits in income tax expense.
Results of Operations
Results of Operations for Year Ended December 31, 2021 Compared to the Year Ended December 31, 2020
The following table summarizes certain information derived from our consolidated statements of operations (in thousands):
Year Ended December 31, |
|
|||||||||||
|
2021 |
|
2020 |
|
$ Change |
|
% Change |
|
||||
Service revenues |
|
$ |
119,065 |
$ |
55,299 |
$ |
63,766 |
|
115.3 |
% |
||
Product revenues |
|
103,543 |
|
23,397 |
|
80,146 |
|
342.5 |
% |
|||
Total revenues |
|
222,608 |
|
78,696 |
|
143,912 |
|
182.9 |
% |
|||
Cost of service revenues |
(69,297) |
(29,521) |
(39,776) |
134.7 |
% |
|||||||
Cost of product revenues |
(56,734) |
(12,841) |
(43,893) |
341.8 |
% |
|||||||
Total cost of revenues |
(126,031) |
(42,362) |
(83,669) |
197.5 |
% |
|||||||
Gross margin |
|
96,577 |
|
36,334 |
|
60,243 |
|
165.8 |
% |
|||
Selling, general and administrative expenses |
|
(97,563) |
|
(56,860) |
|
(40,703) |
|
71.6 |
% |
|||
Engineering and development expenses |
|
(16,843) |
|
(9,484) |
|
(7,359) |
|
77.6 |
% |
|||
Investment income |
3,253 |
761 |
2,492 |
327.2 |
% |
|||||||
Interest expense |
|
(4,689) |
|
(2,560) |
|
(2,129) |
|
83.2 |
% |
|||
Loss on debt extinguishment |
(251,754) |
— |
(251,754) |
100 |
% |
|||||||
Other expense |
|
(2,823) |
|
(929) |
|
(1,894) |
|
203.8 |
% |
|||
Benefit from (provision for) income taxes |
|
(1,686) |
|
45 |
|
(1,731) |
|
(3,874.5) |
% |
|||
|
|
|
|
|
|
|
|
|||||
Net loss |
$ |
(275,528) |
$ |
(32,693) |
$ |
(242,835) |
|
742.8 |
% |
|||
Deemed dividend on Series C convertible preferred stock |
— |
(39,492) |
39,492 |
(100) |
% |
|||||||
Paid-in-kind dividend on Series C convertible preferred stock |
(8,196) |
(2,844) |
(5,352) |
188.2 |
% |
|||||||
Net loss attributable to common stockholders |
$ |
(283,724) |
$ |
(75,029) |
$ |
(208,695) |
|
278.1 |
% |
|||
Total revenues by market
Year Ended December 31, |
||||||||||||
|
2021 |
|
2020 |
|
$ Change |
|
% Change |
|
||||
Pharma/Biopharmaceutical |
$ |
180,203 |
$ |
66,394 |
$ |
113,809 |
|
171.4 |
% |
|||
Animal Health |
|
33,353 |
|
7,846 |
|
25,507 |
|
325.1 |
% |
|||
Human Reproductive Medicine |
|
9,052 |
|
4,456 |
|
4,596 |
|
103.1 |
% |
|||
Total revenues |
$ |
222,608 |
$ |
78,696 |
$ |
143,912 |
|
182.9 |
% |
|||
49
Revenues. Revenues increased $143.9 million, or 182.9%, to $222.6 million for year ended December 31, 2021, as compared to $78.7 million for the year ended December 31, 2020. This increase was primarily driven by revenue from the acquisition of MVE and CRYOPDP on October 1, 2020, which contributed $103.1 million and $59.6 million, respectively. In addition, organic revenue increased by $17.1 million or 39.9%, to $60.0 million for the year ended December 31, 2021, as compared to the same period in 2020, primarily driven by increased activity from our biopharmaceutical customers. During 2021 we experienced an increase in the number of customers utilizing our services and the number of clinical trials we support.
Service revenues increased by $63.8 million, or 115.3%, from $55.3 million to $119.1 million for the year ended December 31, 2021, as compared to the same period in 2020. This increase was driven by the acquisition of CRYOPDP on October 1, 2020, which contributed $46.6 million in revenue from temperature-controlled specialty courier and supply chain services, including packaging, pick-pack kit preparation, the continuing ramp of revenue from our Cryoport Express® solutions, which increased by 16.2 million, or 43.8%, and revenue from our biostorage services, which increased by $0.7 million, or 13.6%.
Product revenues increased by $80.1 million, or 342.5%, from $23.4 million to $103.5 million for the year ended December 31, 2021, as compared to the same period in 2020. The revenues were for the years ending December 31, 2020 and 2021 were primarily a result of the acquisition of MVE Biological Solutions on October 1, 2020, representing revenue from our portfolio of cryogenic stainless-steel freezers, aluminum dewars and related ancillary equipment used in the storage and transport of life sciences commodities, which includes the rapidly growing Cell and Gene Therapy ("C>") market through a global network of distributors and direct client relationships.
Revenues by market
Revenue from the biopharma/pharma market increased $113.8 million, or 171.4%, from $66.4 million to $180.2 million for the year ended December 31, 2021, as compared to the same period in 2020. This increase was driven by revenue from the acquisition of MVE Biological Solutions and CRYOPDP on October 1, 2020, which contributed $67.1 million and $59.6 million, respectively, and the increase in organic revenue by $15.4 million, or 40.4%, to $53.6 million driven by revenue growth from the support of clinical trials. As of December 31, 2021, we supported 602 clinical trials, of which 475 were in the Americas, 93 were in EMEA and 34 were in APAC, compared to 528 clinical trials supported as of December 31, 2020 (419 in the Americas, 84 in EMEA and 25 in APAC). The number of Phase III clinical trials supported increased to 74 trials as of December 31, 2021, of which 51 are in the Americas, 21 are in EMEA, and 2 are in APAC. This compares to 69 Phase III trials (49 in the Americas, 19 in EMEA and 1 in APAC) supported as of December 30, 2020. The activity in the clinical trial space, particularly in the C> market is expected to drive future revenue growth as these clinical trials advance and resulting therapies are commercialized on a global basis.
Our revenue from the animal health market increased $25.5 million, or 325.1%, from $7.8 million to $33.4 million for the year ended December 31, 2021, as compared to the same period in 2020. This increase was primarily driven by the acquisition of MVE Biological Solutions on October 1, 2020.
Revenues in the reproductive medicine market increased $4.6 million, or 103.1%, from 4.5 million to $9.1 million the year ended December 31, 2021, as compared to the same period in 2020. This increase was driven by an increase in organic revenue of $1.5 million, or 39.2%, to $5.3 million, demonstrating the continued success of our CryoStork®-branded offering, and revenue of $3.7 million contributed by MVE Biological Solutions during 2021.
Gross margin and cost of revenues. Gross margin for the year ended December 31, 2021 was 43.4% of total revenues, as compared to 46.2% of total revenues for the year ended December 31, 2020. The decrease in gross margin is impacted by the margin profiles and related margin contributions of the MVE Biological Solutions and CRYOPDP acquisitions completed October 1, 2020. The third quarter 2021 gross margin was impacted by investments in our growth initiatives, as well as increased costs as a result of global supply chain constraints, primarily impacting the MVE Biological Solutions business. Cost of total revenues increased $83.7 million to $126.0 million for the year ended December 31, 2021, as compared to $42.4 million in the same period in 2020. The increase in cost of total revenues is commensurate with the increase in business volume and the addition of cost of revenue from the acquisitions of MVE Biological Solutions and CRYOPDP in October 2020.
Gross margin for our service revenues was 41.8% of service revenues, as compared to 46.6% of service revenues for the year ended December 31, 2020. This decrease was a result of the lower margin contribution from the acquisition of CRYOPDP in October 2020. Our cost of revenues is primarily comprised of freight charges, payroll and associated expenses related to our global logistics and supply chain centers, depreciation expenses of our Cryoport Express® Shippers and supplies and consumables used for our solutions.
50
Gross margin for our product revenues was 45.2% of product revenues, as compared to 45.1% of product revenues for the year ended December 31, 2020. Product revenues, related cost of revenues and resulting gross margins were primarily a result of the acquisition of MVE Biological Solutions. Our cost of product revenues were primarily comprised of materials, direct and indirect labor, inbound freight charges, purchasing and receiving, inspection, and distribution and warehousing of inventory. In addition, shop supplies, facility maintenance costs and depreciation expense for assets used in the manufacturing process were included in cost of product revenues.
Selling, general and administrative expenses. Selling, general and administrative (“SG&A”) expenses include the costs associated with selling our products and services and costs required to support our marketing efforts including legal, accounting, patent, shareholder services, amortization of intangible assets and other administrative functions.
For the year ended December 31, 2021, SG&A expenses increased by $40.7 million, or 71.6% as compared to the same period in 2020. This increase is primarily due to the addition of $36.8 million of SG&A expenses from the acquisition of MVE Biological Solutions and CRYOPDP in October 2020, as well as the continued expansion of our infrastructure to support the acquisitions and expected future growth. Wages and associated employee costs increased $20.3 million from $19.0 million in 2020 to $39.3 million in 2021. Intangible asset amortization expense is included in SG&A and consists of charges related to the amortization of intangible assets associated with the acquisitions of CRYOPDP and MVE Biological Solutions in 2020 and CRYOGENE in 2019, in which we acquired definite-lived intangible assets. Intangible asset amortization expense increased by $7.8 million, from $6.7 million in 2020, to $14.4 million in 2021. Facility and other overhead allocations increased $6.0 million, public company related expenses (including legal, audit and internal control audit fees) increased by $4.2 million and stock compensation expense increased by $4.9 million compared to the same period in 2020. These increases were partially offset by a $9.6 million decrease in consulting, legal and other acquisition-related expenses in 2021 as compared to the same period in 2020.
Engineering and development expenses. Engineering and development expenses increased $7.4 million, or 77.6%, for year ended December 31, 2021, as compared to the same period in 2020. The increase was primarily due to an increase of $3.0 million in consulting, prototype and development costs directed at further enhancing our logistics and supply chain solutions, $2.8 million in wages and associated employee costs to add software development and engineering resources and $0.5 million stock compensation expense. We continually strive to improve and expand the features of our Cryoport Express® Solutions and portfolio of services and suite of temperature-controlled products. Our primary developments are directed towards facilitating the safe, reliable and efficient transport and storage of life science commodities through innovative and technology-based solutions. This includes significantly enhancing our Cryoportal® Logistics Management Platform and related technology solutions as well as developments to expand our Cryoport Express® and Cryoport ELITE™ shipper fleet, such as the Cryosphere™ shipper, a cryogenic dry-vapor shipper utilizing patent pending technology that passively stabilizes the payload through an internal gravitational sphere, thereby further mitigating transport risks. In addition, engineering and development efforts are also focused on MVE Biological Solutions’ portfolio of advanced cryogenic stainless-steel freezers, aluminum dewars and related ancillary equipment used in the storage and transport of life sciences commodities. We supplement our internal engineering and development resources with subject matter experts and consultants to enhance our capabilities and shorten development cycles.
Investment Income. Investment income increased by $2.5 million, for year ended December 31, 2021, as compared to the prior year as a result of higher average invested cash balances offset by lower interest rates on such invested cash balances.
Interest expense. Interest expense increased by $2.1 million, from $2.6 million to $4.7 million for the year ended December 31, 2021, as compared to the prior year due to interest on the convertible senior notes and amortization of the related debt discount.
Loss on extinguishment of debt. The repurchase of the 2025 Senior Notes and issuance of the 2026 Senior Notes were deemed to have substantially different terms based on the present value of the cash flows. Therefore, the repurchase of the 2025 Senior Notes was accounted for as a debt extinguishment, which includes the write off of related deferred financing costs of $2.6 million.
Other expense, net. The increase in other income, net for the year ended December 31, 2021 is primarily due to realized and unrealized gains and losses on short-term investments and foreign currency fluctuations.
51
Provision for income taxes. The provision for income taxes increased $1.7 million for the for year ended December 31, 2021, as compared to the to the same period in 2020. For the year ended December 31, 2021 and 2020, our taxes reflected an effective income tax rate of negative 0.62% and 0.14%, respectively. The negative effective tax rate of 0.62% for the for year ended December 31, 2021 was principally due to our accrual of taxes on our foreign earnings with no offsetting tax benefit for our domestic losses due to the valuation allowance on domestic deferred tax assets. The effective tax rate for the for year ended December 31, 2021 varied significantly from the effective tax rate from the prior year primarily due to our increase in foreign operations as a result of the acquisitions of MVE Biological Solutions and CRYOPDP during the fourth quarter of 2020.
Dividends on Series C preferred convertible stock. Dividends in the aggregate amount of $42.3 million for the year ended December 31, 2020 represent a non-cash deemed dividend in the amount of $39.5 million resulting from a beneficial conversion feature and a paid-in-kind deemed dividend $2.8 million both from the private placement of Series C Preferred Stock with Blackstone as compared to $8.2 million paid-in-kind deemed dividend in 2021.
Non-GAAP Financial Measures
We provide adjusted EBITDA, a non-GAAP financial measure, as a supplemental measure to U.S. GAAP measures regarding our operating performance. Adjusted EBITDA is defined as net loss adjusted for interest expense, income taxes, depreciation and amortization expense, stock-based compensation expense, acquisition and integration costs, inventory step-up charges, loss on extinguishment of debt, investment income, and charges or gains resulting from non-recurring events. Adjusted EBITDA is not calculated in accordance with U.S. GAAP, is not based on any comprehensive set of accounting rules or principles and may be different from non-GAAP financial measures presented by other companies. Non-GAAP financial measures, including adjusted EBIDTA, should not be considered as a substitute for, or superior to, measures of financial performance prepared in accordance with U.S. GAAP.
Management believes adjusted EBITDA provides a useful measure of our operating results, a meaningful comparison with historical results and with the results of other companies, and insight into our ongoing operating performance. Further, management and our board of directors utilize adjusted EBITDA to gain a better understanding of our comparative operating performance from period-to-period and as a basis for planning and forecasting future periods. Management believes adjusted EBITDA, when read in conjunction with our U.S. GAAP financials, is useful to investors because it provides a basis for meaningful period-to-period comparisons of our ongoing operating results, including results of operations, against investor and analyst financial models, identifying trends in our underlying business and performing related trend analyses, and it provides a better understanding of how management plans and measures our underlying business.
A reconciliation of adjusted EBITDA to net loss, the most directly comparable U.S. GAAP financial measure, is presented below.
Cryoport, Inc. and Subsidiaries
Adjusted EBITDA Reconciliation
(Unaudited, in thousands)
Three Months Ended |
Year Ended |
|||||||||||
December 31, |
December 31, |
|||||||||||
|
2021 |
|
2020 |
|
2021 |
|
2020 |
|||||
GAAP net loss |
|
$ |
(260,086) |
|
$ |
(11,530) |
|
(275,528) |
$ |
(32,693) |
||
Non-GAAP adjustments to net loss: |
|
|
|
|
|
|
|
|||||
Depreciation and amortization expense |
|
5,302 |
|
7,370 |
|
20,247 |
|
9,869 |
||||
Acquisition and integration costs |
|
1,066 |
|
3,700 |
|
4,406 |
|
11,163 |
||||
Inventory step-up charges |
|
— |
|
727 |
|
— |
|
727 |
||||
Other non-recurring charges |
|
— |
|
225 |
|
— |
|
225 |
||||
Investment income |
|
(1,636) |
|
(150) |
|
(3,253) |
|
(761) |
||||
Interest expense, net |
|
1,128 |
|
1,077 |
|
4,689 |
|
2,560 |
||||
Loss on extinghishment of debt |
251,754 |
— |
251,754 |
— |
||||||||
Stock-based compensation expense |
|
4,182 |
|
2,561 |
|
15,345 |
|
8,916 |
||||
Income taxes |
|
(876) |
|
(98) |
|
1,686 |
|
(45) |
||||
Adjusted EBITDA |
834 |
3,882 |
|
19,346 |
(39) |
|||||||
52
Liquidity and Capital Resources
As of December 31, 2021, the Company had cash and cash equivalents of $139.1 million, short-term investments of $489.7 million and working capital of $650.9 million. Historically, we have financed our operations primarily through sales of equity securities and debt instruments. We believe that our cash and cash equivalents and short-term investments will be sufficient to fund our operations, including capital expenditures and absent any acquisitions, for at least the next three years.
Cash flows
For the Year Ended December 31, |
|||||||||
2021 |
2020 |
$ Change |
|||||||
(in thousands) |
|||||||||
Operating activities |
|
$ |
8,126 |
|
$ |
(14,866) |
|
$ |
22,992 |
Investing activities |
|
(469,254) |
|
(382,312) |
|
(86,942) |
|||
Financing activities |
|
564,342 |
|
385,585 |
|
178,757 |
|||
Effect of exchange rate changes on cash and cash equivalents |
|
(986) |
|
1,231 |
|
(2,217) |
|||
Net increase in cash and cash equivalents |
$ |
102,228 |
$ |
(10,362) |
$ |
112,590 |
|||
Operating activities
For the year ended December 31, 2021, we provided $8.1 million of cash for operations which included non-cash expenses of $290.6 million comprised of $251.8 million of loss on extinguishment of debt, $20.2 million of depreciation and amortization, $15.3 million of stock-based compensation, $1.4 million of unrealized losses on our equity securities as well as $1.2 million of amortization of debt discount. Also contributing to the cash impact of our net operating loss, excluding non-cash items, was an increase in accounts receivable of $7.3 million, an increase in inventory of $6.0 million, and an increase in accrued compensation of $2.5 million. These increases were partially offset by the net loss of $275.5 million and a decrease in accounts payable and accrued expenses of $0.3 million and a decrease in prepaids and other current assets of $3.1 million.
Investing activities
Net cash used in investing activities of $469.3 million during the year ended December 31, 2021 was primarily due to the $482.7 million purchase of short-term investments, the acquisition of CTSA and F-airGate for $5.0 million, $24.8 million for the capitalization of software development costs for our Cryoportal® Logistics Management Platform and additional purchases of Cryoport Express® Shippers, Smart Pak IITM Condition Monitoring Systems, freezers and computer equipment, partially offset by the maturity of short-term investments of $44.0 million.
Financing Activity
Net cash provided by financing activities during the year ended December 31, 2021 totaled $564.3 million during the year ended December 31, 2021, primarily as a result of net proceeds of $389.9 million from the issuance of 2026 convertible senior notes issued in November 2021, $248.9 million in net proceeds from the November 2021 direct placement of common stock, $269.8 million in net proceeds from our February 2021 public offering of common stock and $9.0 million proceeds from the exercise of stock options and warrants, partially offset by the $349.8 million repayment of the 2025 convertible senior notes.
53
Convertible Senior Notes
2026 Senior Notes
In November 2021, the Company issues $402.5 million principal amount of its 0.75% Convertible Senior Notes due 2026. The 2026 Senior Notes issued include $52.5 million principal amount issued pursuant to the full exercise by the initial purchasers of their option to purchase additional 2026 Senior Notes provided by the purchase agreement between the Company and the initial purchasers of the 2025 Senior Notes. The Company received $390.4 million in net proceeds from the offering, after deducting underwriting discounts and commission of $12.1 million and incurred approximately $0.6 million of third party offering related costs. The 2026 Senior Notes will accrue interest at a rate of 0.75% per annum, payable semi-annually in arrears on June 1 and December 1 of each year, beginning on June 1, 2022 and will mature on December 1, 2026, unless earlier repurchased, redeemed, or converted in accordance with the terms of the 2026 Senior Notes.
2025 Senior Notes
In May 2020, the Company issued $115.0 million aggregate principal amount of 3.00% convertible senior notes due in 2025, which includes the initial purchasers' exercise in full of their option to purchase an additional $15.0 million principal amount of the 2025 Senior Notes, in a private placement exempt from registration under the Securities Act. The Company received $111.3 million from the offering, net of underwriting discounts and commissions of $3.7 million, and incurred approximately $0.3 million in third-party offering related costs. The 2025 Senior Notes bear cash interest at a rate of 3.00%, payable semi-annually on June 1 and December 1 of each year, beginning on December 1, 2020 and will mature on June 1, 2025, unless earlier repurchased, redeemed, or converted in accordance with the terms of the 2025 Senior Notes. On November 9, 2021, the Company entered into separate, privately negotiated note purchase agreements with a limited number of holders of its 2025 Senior Notes pursuant to which the Company repurchased approximately $100.7 million principal amount of 2025 Senior Notes for an aggregate cash repurchase price of approximately $351.1 million, which includes accrued and unpaid interest on the repurchased 2025 Senior Notes.
The Convertible Senior Notes comprise the Company’s senior, unsecured obligations and are (i) equal in right of payment with the Company’s existing and future senior, unsecured indebtedness; (ii) senior in right of payment to the Company’s existing and future indebtedness that is expressly subordinated to the Convertible Senior Notes; (iii) effectively subordinated to the Company’s existing and future secured indebtedness, to the extent of the value of the collateral securing that indebtedness; and (iv) structurally subordinated to all existing and future indebtedness and other liabilities, including trade payables, and (to the extent the Company is not a holder thereof) preferred equity, if any, of the Company’s subsidiaries. See Note 10: “Convertible Senior Notes” of our accompanying consolidated financial statements for additional information.
November 2021 Registered Direct Placement and Stock Purchase Agreements
Concurrent with the issuance of the 2026 Senior Notes in November 2021, the Company conducted a registered direct placement of 3,072,038 shares of its common stock at $81.10/share (“Concurrent Placement”). The Company used the net proceeds from the Concurrent Placement, together with a portion of the net proceeds from the issuance of the 2026 Senior Notes, to repurchase approximately $100.7 million principal amount of the 2025 Senior Notes in separate, privately negotiated repurchase transactions with a limited number of holders of the 2025 Senior Notes, for a cash repurchase price of approximately $351.1 million. The remainder of the net proceeds of approximately $288.4 million, after deducting banker fees, will be used for general corporate purposes. See Note 10: “Convertible Senior Notes” of our accompanying consolidated financial statements for additional information.
54
Blackstone Private Placement
In connection with the MVE Acquisition, on October 1, 2020, the Company completed a private placement with an investment vehicle of funds affiliated with The Blackstone Group Inc., consisting of (i) 250,000 shares of a newly designated 4.0% Series C Convertible Preferred Stock, par value $0.001 per share (“Series C Preferred Stock”), at a price of $1,000 per share, for $250.0 million, and (ii) 675,536 shares of common stock of the Company, par value $0.001 per share for $25.0 million, for an aggregate purchase price of $275.0 million. The net proceeds of this transaction were $263.6 million. The Series C Preferred Stock ranks senior to the shares of the Company’s common stock, with respect to dividend rights and rights upon the voluntary or involuntary liquidation, dissolution, or winding up of the affairs of the Company. On February 5, 2021, the Company received a waiver and conversion notice from Blackstone Freeze Parent L.P. and Blackstone Tactical Opportunities Fund – FD L.P. and converted an aggregate of 50,000 shares of Series C Convertible Preferred Stock, resulting in the issuance of an aggregate of 1,312,860 shares of common stock. See Note 15: “Stockholders’ Equity—Blackstone Private Placement” of our accompanying consolidated financial statements for additional information.
June 2019 Public Offering
In June 2019, the Company completed an underwritten public offering of 4,312,500 shares of its common stock. The shares were issued and sold pursuant to an underwriting agreement, dated June 19, 2019, by and among the Company, on the one hand, and Jefferies LLC and SVB Leerink LLC, as representatives of certain underwriters at a public offering price per share of $17.00, before deducting underwriting discounts and commissions. The shares include 562,500 shares issued and sold pursuant to the Underwriters’ exercise in full of their option to purchase additional shares of common stock pursuant to the Underwriting Agreement. The Company received net proceeds of approximately $68.8 million from the offering after deducting underwriting discounts and commissions and estimated offering expenses payable by the Company.
The Company's management believes that Cryoport's current cash and cash equivalents and short-term investments will be sufficient to fund its operations, including capital expenditures and absent any acquisitions, for at least the next three years. Management recognizes that the Company may need to obtain additional capital in the future to fund its operations until sustained profitable operations are achieved. Additional funding plans may include obtaining additional capital through equity and/or debt funding sources. No assurance can be given that additional capital, if needed, will be available when required or upon terms acceptable to the Company.
Off-Balance Sheet Arrangements
We do not have any off-balance sheet arrangements within the meaning of Item 303(a)(4) of Regulation S-K.
Impact of Inflation
Inflation generally impacts us by increasing our costs of labor, material, transportation and pricing from third party manufacturers. While the rates of inflation have not had a material impact on our financial statements in the past, we have seen some impact on gross margins during the second half of 2021. Based on the current economic outlook, inflationary pressures could affect our financial performance in the future if cost increases cannot be offset by net realized annual price increases and productivity gains.
Item 7A. Quantitative and Qualitative Disclosures About Market Risk
We are exposed to market risk for the effect of interest rate changes, foreign currency fluctuations, and changes in the market values of our investments.
55
Interest Rate Risk
Our exposure to market risk for changes in interest rates relates primarily to our investment portfolio and our long-term debt. Our long-term debt is carried at amortized cost and fluctuations in interest rates do not impact our consolidated financial statements. However, the fair value of our debt, which pays interest at a fixed rate, will generally fluctuate with movements of interest rates, increasing when interest rates are declining and declining when interest rates are increasing. We invest our excess cash in high investment grade money market funds and investment grade short to intermediate-term fixed income securities. Fixed income securities may have their fair market value adversely affected due to a rise in interest rates, and we may suffer losses if forced to sell securities that have declined in market value due to changes in interest rates. As of December 31, 2021, the estimated fair value of the Convertible Senior Notes was $345.4 million. For additional information about the Convertible Senior Notes, see Note 10 in our accompanying consolidated financial statements.
Foreign Exchange Risk
We operate in the United States and other foreign countries, which creates exposure to foreign currency exchange fluctuations. Net sales and related expenses generated from our international business are primarily denominated in the functional currencies of the corresponding subsidiaries and primarily include Euros, British Pounds, Chinese Yuan, and Indian Rupee.
We have foreign exchange risk related to foreign-denominated cash and cash equivalents. Based on the balance of as of December 31, 2021, of $19.3 million, an assumed 5%, 10%, and 20% adverse change to foreign exchange would result in declines of $1.0 million, $1.9 million and $3.9 million, respectively, recorded in “Accumulated other comprehensive income (loss)”, a separate component of stockholders’ equity.
We have foreign exchange risk related to our long-term intercompany balances denominated in Euros. Based on the long-term intercompany balances as of December 31, 2021, an assumed 5%, 10%, and 20% adverse change to foreign exchange would result in losses of $3.9 million, $7.8 million and $15.7 million, respectively, recorded to “Accumulated other comprehensive income (loss)”.
Item 8. Financial Statements and Supplementary Data
Our annual consolidated financial statements are included in Part IV, Item 15 of this Form 10-K and are incorporated into this Item 8 by reference.
Item 9. Changes in and Disagreements with Accountants on Accounting and Financial Disclosure
None.
Item 9A. Controls and Procedures
(a) Evaluation of Disclosure Controls and Procedures
The term “disclosure controls and procedures” (as defined in Rule 13a-15(e) under the Exchange Act) refers to the controls and other procedures of a company that are designed to ensure that information required to be disclosed by a company in the reports that it files or submits under the Exchange Act is recorded, processed, summarized and reported within the time periods specified in the SEC’s rules and forms. Disclosure controls and procedures include, without limitation, controls and procedures designed to ensure that information required to be disclosed by a company in the reports that it files or submits under the Exchange Act is accumulated and communicated to the company’s management, including its principal executive and principal financial officers, or persons performing similar functions, as appropriate to allow timely decisions regarding required disclosure. Under the supervision and with the participation of our management, including our Chief Executive Officer and Chief Financial Officer, we have conducted an evaluation of the effectiveness of the design and operation of our disclosure controls and procedures as of December 31, 2021. Based on this evaluation, our Chief Executive Officer and Chief Financial Officer concluded that our disclosure controls and procedures were effective as of December 31, 2021.
56
(b) Management’s Report on Internal Control Over Financial Reporting.
Management’s Report on Internal Control Over Financial Reporting which appears on the following page is incorporated herein by reference.
Ernst & Young LLP, an independent registered public accounting firm, has audited the effectiveness of our internal control over financial reporting as of December 31, 2021, as stated in its attestation report included in Part II, Item 8. “Financial Statements and Supplementary Data” included elsewhere in this Form 10-K.
(c) Changes In Internal Control Over Financial Reporting
During the quarter ended December 31, 2021, the Company continued the process of integrating into its operations and internal control processes the acquired operations of MVE Biological Solutions and CRYOPDP, resulting in some of the acquired operations’ historical internal control over financial reporting being modified or superseded by the Company’s internal control over financial reporting. There were no other changes in our internal control over financial reporting during the quarter ended December 31, 2021 that have materially affected, or are reasonably likely to materially affect, our internal control over financial reporting.
Item 9B. Other Information
None.
Item 9C. Disclosure Regarding Foreign Jurisdictions that Prevent Inspections
Not applicable.
57
CRYOPORT, INC.
MANAGEMENT’S REPORT ON
INTERNAL CONTROL OVER FINANCIAL REPORTING
The management of the Company is responsible for establishing and maintaining effective internal control over financial reporting (as defined in Rule 13a-15(f) under the Exchange Act) and for the assessment of the effectiveness of internal control over financial reporting. The Company’s internal control over financial reporting is a process designed to provide reasonable assurance regarding the reliability of financial reporting and the preparation of consolidated financial statements for external purposes in accordance with accounting principles generally accepted in the United States of America.
The Company’s internal control over financial reporting is supported by written policies and procedures that:
| ● | pertain to the maintenance of records that, in reasonable detail, accurately and fairly reflect the transactions and dispositions of the Company’s assets; |
| ● | provide reasonable assurance that transactions are recorded as necessary to permit preparation of consolidated financial statements in accordance with accounting principles generally accepted in the United States of America, and that receipts and expenditures of the Company are being made only in accordance with authorizations of the Company’s management and directors; and |
| ● | provide reasonable assurance regarding prevention or timely detection of unauthorized acquisition, use or disposition of the Company’s assets that could have a material effect on the consolidated financial statements. |
Because of its inherent limitations, internal control over financial reporting may not prevent or detect misstatements. Projections of any evaluation of effectiveness to future periods are subject to the risk that controls may become inadequate because of changes in conditions, or that the degree of compliance with the policies or procedures may deteriorate.
In connection with the preparation of the Company’s annual consolidated financial statements, management of the Company has undertaken an assessment of the effectiveness of the Company’s internal control over financial reporting based on criteria established in Internal Control — Integrated Framework (2013) issued by the Committee of Sponsoring Organizations of the Treadway Commission. Management’s assessment included an evaluation of the design of the Company’s internal control over financial reporting and testing of the operational effectiveness of the Company’s internal control over financial reporting.
Based on this assessment, management has concluded that the Company’s internal control over financial reporting was effective as of December 31, 2021.
By: |
/s/ JERRELL W. SHELTON |
|
|
Jerrell W. Shelton, |
|
|
Chief Executive Officer and Director |
|
By: |
/s/ ROBERT STEFANOVICH |
|
|
Robert Stefanovich, |
|
|
Chief Financial Officer |
|
February 28, 2022
58
PART III
Item 10. Directors, Executive Officers and Corporate Governance
A list of our executive officers and their respective biographical information appears in Part I, Item 1 of this Form 10-K.
We have adopted a corporate code of conduct that applies to our directors and all employees, including our Chief Executive Officer and Chief Financial Officer. We have posted the text of our corporate code of conduct on our website at www.cryoport.com on the “Investor Relations: Corporate Governance” page under the heading “Governance Documents”. We intend to satisfy the requirement under Item 5.05 of Form 8-K regarding disclosure of amendments to, or waivers from, provisions of our corporate code of conduct by posting such information on our website.
The other information required under this item is incorporated by reference from our definitive proxy statement related to our 2022 Annual Meeting of Stockholders, or the Proxy Statement, to be filed with the SEC within 120 days of our fiscal year ended December 31, 2021.
Item 11. Executive Compensation
The information required by this item can be found in our Proxy Statement and is incorporated herein by reference.
Item 12. Security Ownership of Certain Beneficial Owners and Management and Related Stockholder Matters
The information required by this item can be found in our Proxy Statement and is incorporated herein by reference.
Item 13. Certain Relationships and Related Transactions, and Director Independence
The information required by this item can be found in our Proxy Statement and is incorporated herein by reference.
Item 14. Principal Accountant Fees and Services
The information required by this item can be found in our Proxy Statement and is incorporated herein by reference.
59
PART IV
Item 15. Exhibits and Financial Statement Schedules
(a)(1) Consolidated Financial Statements:
(a)(2) Financial Statement Schedules: All financial statement schedules are omitted because they are not applicable or the required information is included in the Consolidated Financial Statements or notes thereto.
(a)(3) Exhibits.
60
Index to Exhibits
Exhibit No. |
|
Description |
|
|---|---|---|---|
2.1˄ |
|||
2.2˄ |
|||
|
|
||
3.1 |
|||
|
|
||
3.2 |
|||
3.3 |
|||
|
|
||
3.4 |
|||
|
|
||
3.5 |
|||
|
|
||
3.6 |
|||
|
|
||
3.7 |
|||
|
|
||
3.8 |
|||
|
|
||
3.9 |
|||
|
|
||
3.10 |
|||
|
|
||
3.11 |
|||
|
|
||
3.12 |
|||
|
|||
3.13 |
|||
|
|
||
4.1 |
|||
|
|
||
4.2 |
|||
|
|
61
Exhibit No. |
|
Description |
|
|---|---|---|---|
4.3 |
|||
4.4 |
|||
4.5 |
|||
10.1* |
|||
|
|
||
10.2* |
|||
|
|||
10.3* |
|||
|
|
||
10.4* |
|||
|
|
||
10.5* |
|||
|
|
||
10.6* |
|||
|
|
||
10.7* |
|||
|
|
||
10.8* |
|||
10.9* |
|||
|
|
||
10.10* |
|||
|
|
||
10.11* |
|||
|
|
||
10.12 |
|||
|
|
||
10.13 |
|||
|
|||
10.14˄ |
|||
|
62
Exhibit No. |
|
Description |
|
|---|---|---|---|
10.15 |
|||
|
|||
10.16 |
|||
|
|
||
21+ |
|||
|
|
||
23.1+ |
Consent of Ernst & Young LLP, Independent Registered Public Accounting Firm. |
||
|
|
||
31.1+ |
|||
|
|
||
31.2+ |
|||
32.1+ |
|||
|
|
||
32.2+ |
|||
|
|
||
101.INS+ |
Inline XBRL Instance Document - the instance document does not appear in the Interactive Data File because its XBRL tags are embedded within the Inline XBRL document. |
||
|
|
||
101.SCH+ |
Inline XBRL Taxonomy Extension Schema Document. |
||
|
|
||
101.CAL+ |
Inline XBRL Taxonomy Extension Calculation Linkbase Document. |
||
|
|
||
101.DEF+ |
Inline XBRL Taxonomy Extension Definition Linkbase Document. |
||
|
|
||
101.LAB+ |
Inline XBRL Taxonomy Extension Label Linkbase Document. |
||
|
|
||
101.PRE+ |
Inline XBRL Taxonomy Extension Presentation Linkbase Document. |
||
|
|||
104 |
Cover Page Interactive Data File (formatted as Inline XBRL and contained in Exhibit 101). |
˄Certain exhibits and schedules have been omitted pursuant to Item 601(b)(2) or Item 601(a)(5) of Regulation S-K. The Company hereby undertakes to furnish copies of such omitted materials supplementally upon request by the SEC.
* |
Indicates a management contract or compensatory plan or arrangement. |
+ |
Filed or furnished herewith. |
Item 16. Form 10-K Summary
None.
63
SIGNATURES
Pursuant to the requirements of Section 13 or 15(d) of the Securities Exchange Act of 1934, the registrant has duly caused this Annual Report on Form 10-K to be signed on its behalf by the undersigned, thereunto duly authorized.
|
Cryoport, Inc. |
|
|
|
|
|
By: |
/s/ JERRELL W. SHELTON |
|
|
Jerrell W. Shelton |
|
|
Chief Executive Officer and Director |
Date: February 28, 2022
Pursuant to the requirements of the Securities Exchange Act of 1934, this Annual Report on Form 10-K has been signed below by the following persons on behalf of the registrant and in the capacities and on the dates indicated:
Signature |
|
Title |
|
Date |
|
|
|
|
|||
/s/ JERRELL W. SHELTON |
Chief Executive Officer and Director |
February 28, 2022 |
|||
Jerrell W. Shelton |
(Principal Executive Officer) |
||||
|
|
||||
/s/ ROBERT S. STEFANOVICH |
Chief Financial Officer |
February 28, 2022 |
|||
Robert S. Stefanovich |
(Principal Financial and Accounting Officer) |
||||
|
|
||||
/s/ RICHARD BERMAN |
Director |
February 28, 2022 |
|||
Richard Berman |
|
||||
|
|
||||
/s/ DANIEL M. HANCOCK |
Director |
February 28, 2022 |
|||
Daniel M. Hancock |
|
||||
|
|
||||
/s/ ROBERT HARIRI, M.D., PH.D. |
Director |
February 28, 2022 |
|||
Robert Hariri, M.D., Ph.D. |
|
||||
|
|
||||
/s/ RAMKUMAR MANDALAM, PH.D. |
Director |
February 28, 2022 |
|||
Ramkumar Mandalam, Ph.D. |
|
||||
|
|
||||
/s/ EDWARD ZECCHINI |
Director |
February 28, 2022 |
|||
Edward Zecchini |
|
||||
|
|||||
/s/ RAM JAGANNATH |
Director |
February 28, 2022 |
|||
Ram Jagannath |
|
||||
/s/ LINDA BADDOUR |
Director |
February 28, 2022 |
|||
Linda Baddour |
64
Cryoport, Inc. and Subsidiaries
Consolidated Financial Statements
As of December 31, 2021 and 2020
Years Ended December 31, 2021, 2020 and 2019
Cryoport, Inc. and Subsidiaries
Consolidated Financial Statements
INDEX TO FINANCIAL STATEMENTS
F-1
Report of Independent Registered Public Accounting Firm
To the Stockholders and the Board of Directors of Cryoport, Inc.
Opinion on the Financial Statements
We have audited the accompanying consolidated balance sheets of Cryoport, Inc. and subsidiaries (the Company) as of December 31, 2021 and 2020, the related consolidated statements of operations, comprehensive loss, stockholders' equity and cash flows for each of the three years in the period ended December 31, 2021, and the related notes (collectively referred to as the “consolidated financial statements”). In our opinion, the consolidated financial statements present fairly, in all material respects, the financial position of the Company at December 31, 2021 and 2020, and the results of its operations and its cash flows for each of the three years in the period ended December 31, 2021, in conformity with U.S. generally accepted accounting principles.
We also have audited, in accordance with the standards of the Public Company Accounting Oversight Board (United States) (PCAOB), the Company’s internal control over financial reporting as of December 31, 2021, based on criteria established in Internal Control-Integrated Framework issued by the Committee of Sponsoring Organizations of the Treadway Commission (2013 framework), and our report dated February 28, 2022 expressed an unqualified opinion thereon.
Basis for Opinion
These financial statements are the responsibility of the Company's management. Our responsibility is to express an opinion on the Company’s financial statements based on our audits. We are a public accounting firm registered with the PCAOB and are required to be independent with respect to the Company in accordance with the U.S. federal securities laws and the applicable rules and regulations of the Securities and Exchange Commission and the PCAOB.
We conducted our audits in accordance with the standards of the PCAOB. Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement, whether due to error or fraud. Our audits included performing procedures to assess the risks of material misstatement of the financial statements, whether due to error or fraud, and performing procedures that respond to those risks. Such procedures included examining, on a test basis, evidence regarding the amounts and disclosures in the financial statements. Our audits also included evaluating the accounting principles used and significant estimates made by management, as well as evaluating the overall presentation of the financial statements. We believe that our audits provide a reasonable basis for our opinion.
Critical Audit Matter
The critical audit matter communicated below is a matter arising from the current period audit of the financial statements that was communicated or required to be communicated to the audit committee and that: (1) relates to accounts or disclosures that are material to the financial statements and (2) involved our especially challenging, subjective or complex judgments. The communication of the critical audit matter does not alter in any way our opinion on the consolidated financial statements, taken as a whole, and we are not, by communicating the critical audit matter below, providing a separate opinion on the critical audit matter or on the accounts or disclosures to which it relates.
F-2
Accounting for Convertible Notes |
||
Description of the Matter |
As described in Note 10 to the consolidated financial statements, the Company issued $402.5 million aggregate principal of convertible notes in November 2021 (“2026 Senior Notes”). The Company used a portion of the proceeds from the issuance of the 2026 Senior Notes, along with net proceeds from a registered direct placement of its common stock, to redeem $100.7 million principal amount of its convertible notes (“2025 Senior Notes”) through privately negotiated repurchase agreements. The Company evaluated the transaction for modification or extinguishment accounting and recorded an extinguishment loss of $251.8 million related to the settlement of the 2025 Notes. Auditing the accounting treatment for the 2026 Senior Notes and the convertible debt exchange involved especially challenging and complex auditor judgment and required team members with specialized knowledge and skills in accounting and in evaluating the Company’s assessment of the terms of the 2026 Senior Notes to determine the existence of any derivatives, the Company’s accounting assessment related to the extinguishment of the 2025 Senior Notes, and the calculation of the related loss on the settlement of the 2025 Senior Notes. |
|
How We Addressed the Matter in Our Audit |
We obtained an understanding, evaluated the design and tested the operating effectiveness of the Company’s controls over the issuance of the 2026 Senior Notes and the accounting for the debt exchange, including the determination of extinguishment accounting for the 2025 Senior Notes. Our testing of the Company’s accounting for the issuance of the 2026 Senior Notes included, among other procedures, reading the underlying agreement to determine if any derivatives exist, and evaluating the Company’s accounting analysis and recording of the notes. To test the convertible debt exchange transaction, we evaluated the Company’s accounting assessment, including the cash flow test used in the determination of the accounting treatment as an extinguishment of debt and we tested the Company’s loss on debt extinguishment calculation. We also evaluated the appropriateness of the Company’s disclosures. |
/s/
We have served as the Company’s auditor since 2019.
February 28, 2022
F-3
Report of Independent Registered Public Accounting Firm
To the Stockholders and the Board of Directors of Cryoport, Inc.
Opinion on Internal Control Over Financial Reporting
We have audited the accompanying consolidated balance sheets of Cryoport, Inc. and subsidiaries (the Company) as of December 31, 2021 and 2020, the related consolidated statements of operations, comprehensive loss, stockholders' equity and cash flows for each of the three years in the period ended December 31, 2021, and the related notes (collectively referred to as the “consolidated financial statements”). In our opinion, the consolidated financial statements present fairly, in all material respects, the financial position of the Company at December 31, 2021 and 2020, and the results of its operations and its cash flows for each of the three years in the period ended December 31, 2021, in conformity with U.S. generally accepted accounting principles.
We also have audited, in accordance with the standards of the Public Company Accounting Oversight Board (United States) (PCAOB), the Company's internal control over financial reporting as of December 31, 2021, based on criteria established in Internal Control-Integrated Framework issued by the Committee of Sponsoring Organizations of the Treadway Commission (2013 framework), and our report dated February 28, 2022 expressed an unqualified opinion thereon.
Basis for Opinion
The Company’s management is responsible for maintaining effective internal control over financial reporting and for its assessment of the effectiveness of internal control over financial reporting included in the accompanying Management’s Report on Internal Control over Financial Reporting. Our responsibility is to express an opinion on the Company’s internal control over financial reporting based on our audit. We are a public accounting firm registered with the PCAOB and are required to be independent with respect to the Company in accordance with the U.S. federal securities laws and the applicable rules and regulations of the Securities and Exchange Commission and the PCAOB.
We conducted our audit in accordance with the standards of the PCAOB. Those standards require that we plan and perform the audit to obtain reasonable assurance about whether effective internal control over financial reporting was maintained in all material respects.
Our audit included obtaining an understanding of internal control over financial reporting, assessing the risk that a material weakness exists, testing and evaluating the design and operating effectiveness of internal control based on the assessed risk, and performing such other procedures as we considered necessary in the circumstances. We believe that our audit provides a reasonable basis for our opinion.
Definition and Limitations of Internal Control Over Financial Reporting
A company’s internal control over financial reporting is a process designed to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles. A company’s internal control over financial reporting includes those policies and procedures that (1) pertain to the maintenance of records that, in reasonable detail, accurately and fairly reflect the transactions and dispositions of the assets of the company; (2) provide reasonable assurance that transactions are recorded as necessary to permit preparation of financial statements in accordance with generally accepted accounting principles, and that receipts and expenditures of the company are being made only in accordance with authorizations of management and directors of the company; and (3) provide reasonable assurance regarding prevention or timely detection of unauthorized acquisition, use, or disposition of the company’s assets that could have a material effect on the financial statements.
Because of its inherent limitations, internal control over financial reporting may not prevent or detect misstatements. Also, projections of any evaluation of effectiveness to future periods are subject to the risk that controls may become inadequate because of changes in conditions, or that the degree of compliance with the policies or procedures may deteriorate.
/s/ Ernst & Young LLP
Irvine, California
February 28, 2022
F-4
Cryoport, Inc. and Subsidiaries
Consolidated Balance Sheets
(in thousands, except share data)
December 31, |
||||||
|
2021 |
|
2020 |
|||
ASSETS |
||||||
Current Assets: |
|
|
||||
Cash and cash equivalents |
$ |
|
$ |
|
||
Short-term investments |
|
|
|
|
||
Accounts receivable, net of allowance for doubtful accounts of $ |
|
|
|
|
||
Inventories |
|
|
|
|
||
Prepaid expenses and other current assets |
|
|
|
|
||
Total current assets |
|
|
|
|
||
Property and equipment, net |
|
|
|
|||
Operating lease right-of-use assets |
|
|
||||
Intangible assets, net |
|
|
|
|||
Goodwill |
|
|
||||
Deposits |
|
|
|
|||
Other long-term assets |
|
|
||||
Total assets |
$ |
|
$ |
|
||
LIABILITIES AND STOCKHOLDERS’ EQUITY |
|
|
|
|
||
Current Liabilities: |
|
|
|
|
||
Accounts payable and other accrued expenses |
$ |
|
$ |
|
||
Accrued compensation and related expenses |
|
|
|
|||
Deferred revenue |
|
|
|
|||
Operating lease liabilities |
|
|
||||
Finance lease liabilities |
|
|
|
|||
Total current liabilities |
|
|
|
|
||
Convertible senior notes, net of discount of $ |
|
|
||||
Note payable, net of discount of $ |
|
|
||||
Operating lease liabilities, net of current portion |
|
|
||||
Finance lease liabilities, net of current portion |
|
|
||||
Deferred tax liability |
|
|
||||
Other long-term liabilities |
|
|
||||
Contingent consideration |
|
|
||||
Total liabilities |
|
|
|
|
||
Commitments and contingencies |
|
|
|
|
||
Stockholders’ Equity: |
|
|
|
|
||
Preferred stock, $ |
|
|
|
|
||
Class A convertible preferred stock, $ |
|
|
|
|
||
Class B convertible preferred stock, $ |
|
|
|
|
||
Class C convertible preferred stock, $ |
|
|
||||
Common stock; $ |
|
|
||||
Additional paid-in capital |
|
|
|
|
||
Accumulated deficit |
|
( |
|
( |
||
Accumulated other comprehensive income (loss) |
|
( |
|
|
||
Total stockholders’ equity |
|
|
|
|
||
Total liabilities and stockholders’ equity |
$ |
|
$ |
|
||
See accompanying notes to consolidated financial statements.
F-5
Cryoport, Inc. and Subsidiaries
Consolidated Statements of Operations
(in thousands, except per share data)
Years Ended |
||||||||||
December 31, |
||||||||||
|
|
2021 |
|
2020 |
|
2019 |
|
|||
Service revenues |
$ |
|
$ |
|
$ |
|
||||
Product revenues |
|
|
— |
|||||||
Total revenues |
|
|
|
|||||||
Cost of service revenues |
|
|
|
|||||||
Cost of product revenues |
|
|
|
|
— |
|||||
Total cost of revenues |
|
|
|
|||||||
Gross margin |
|
|
|
|
|
|||||
|
|
|
|
|||||||
Operating costs and expenses: |
|
|
|
|
|
|
||||
Selling, general and administrative |
|
|
|
|
|
|
||||
Engineering and development |
|
|
|
|
|
|
||||
Total operating costs and expenses |
|
|
|
|
|
|
||||
|
|
|
|
|||||||
Loss from operations |
|
( |
|
( |
|
( |
||||
Other income (expense): |
|
|
|
|
|
|
||||
Investment income |
|
|
|
|||||||
Interest expense |
|
( |
|
( |
|
( |
||||
Loss on debt extinguishment |
( |
— |
— |
|||||||
Other income (expense), net |
|
( |
|
( |
|
|
||||
Total other expense, net |
( |
|
( |
( |
||||||
Loss before provision for income taxes |
|
( |
|
( |
|
( |
||||
(Provision for) benefit from income taxes |
|
( |
|
|
|
( |
||||
Net loss |
( |
$ |
( |
$ |
( |
|||||
Deemed dividend on Series C convertible preferred stock |
— |
( |
— |
|||||||
Paid-in-kind dividend on Series C convertible preferred stock |
( |
( |
— |
|||||||
Net loss attributable to common stockholders |
( |
$ |
( |
$ |
( |
|||||
Net loss per share attributable to common stockholders– basic and diluted |
( |
( |
( |
|||||||
Weighted average common shares outstanding – basic and diluted |
|
|
|
|
|
|
||||
See accompanying notes to consolidated financial statements.
F-6
Cryoport, Inc. and Subsidiaries
Consolidated Statements of Comprehensive Loss
(in thousands)
Years Ended December 31, |
|||||||||
|
2021 |
|
2020 |
|
2019 |
||||
Net loss |
$ |
( |
$ |
( |
$ |
( |
|||
Other comprehensive income (loss), net of tax: |
|
|
|
|
|
|
|||
Net unrealized gain (loss) on available-for-sale debt securities |
|
( |
|
|
|
( |
|||
Reclassification of realized gain on available-for-sale debt securities to earnings |
( |
( |
( |
||||||
Foreign currency translation adjustments |
|
( |
|
|
|
|
|||
Other comprehensive income (loss) |
|
( |
|
|
|
( |
|||
Total comprehensive loss |
$ |
( |
$ |
( |
$ |
( |
|||
See accompanying notes to consolidated financial statements.
F-7
Cryoport, Inc. and Subsidiaries
Consolidated Statements of Stockholders’ Equity
(In thousands, except share data)
Accumulated |
||||||||||||||||||||||||||||||||
Other |
||||||||||||||||||||||||||||||||
Class A |
Class B |
Class C |
Comprehensive |
Total |
||||||||||||||||||||||||||||
Preferred Stock |
Preferred Stock |
Preferred Stock |
Common Stock |
Additional |
Accumulated |
Income |
Stockholders' |
|||||||||||||||||||||||||
|
Shares |
|
Amount |
|
Shares |
|
Amount |
|
Shares |
Amount |
|
Shares |
|
Amount |
|
Paid–In Capital |
|
Deficit |
|
(Loss) |
|
Equity (Deficit) |
||||||||||
|
|
|
|
|
|
|
|
|
|
|||||||||||||||||||||||
Balance at December 30, 2018 |
|
|
$ |
|
|
|
$ |
|
|
$ |
|
|
|
$ |
|
$ |
|
$ |
( |
$ |
|
$ |
|
|||||||||
Net loss |
|
|
|
|
|
|
|
|
— |
— |
|
|
|
|
|
|
|
( |
|
|
|
( |
||||||||||
Other comprehensive loss, net of taxes |
|
|
|
|
|
|
|
|
— |
— |
|
|
|
|
|
|
|
|
|
( |
|
( |
||||||||||
Stock-based compensation expense |
|
|
|
|
|
|
|
|
— |
— |
|
|
|
|
|
|
|
|
|
|
|
|
||||||||||
Accelerated stock-based compensation expense |
|
|
|
|
|
|
|
|
— |
— |
|
|
|
|
|
|
|
|
|
|
|
|
||||||||||
Proceeds from public offering net of costs of $ |
|
|
|
|
|
|
|
|
— |
— |
|
|
|
|
|
|
|
|
|
|
|
|
||||||||||
Issuance of common stock for convertible debt and accrued interest |
|
|
|
|
|
|
|
|
— |
— |
|
|
|
|
|
|
|
|
|
|
|
|
||||||||||
Issuance of common stock for board of director compensation |
|
|
|
|
|
|
|
|
— |
— |
|
|
|
|
|
|
|
|
|
|
|
|
||||||||||
Proceeds from exercise of common stock options and warrants |
|
|
|
|
|
|
|
|
— |
— |
|
|
|
|
|
|
|
|
|
|
|
|
||||||||||
Balance at December 31, 2019 |
|
|
$ |
|
|
|
$ |
|
|
$ |
|
|
|
$ |
|
$ |
|
$ |
( |
$ |
( |
$ |
|
|||||||||
Net loss |
|
|
|
|
|
|
|
|
— |
— |
|
|
|
|
|
|
|
( |
|
|
|
( |
||||||||||
Other comprehensive income, net of taxes |
|
|
|
|
|
|
|
|
— |
— |
|
|
|
|
|
|
|
|
|
|
|
|
||||||||||
Stock-based compensation expense |
|
|
|
|
|
|
|
|
— |
— |
|
|
|
|
|
|
|
|
|
|
|
|
||||||||||
Issuance of common stock for board of director compensation |
|
|
|
|
— |
— |
|
|
|
|
|
|
||||||||||||||||||||
Issuance of common stock in private placement, net of costs of $ |
|
|
|
|
— |
— |
|
|
|
|
|
|
||||||||||||||||||||
Issuance of Series C convertible preferred stock in private placement, net of costs of $ |
|
|
|
|
|
— |
|
|
|
|
|
|
||||||||||||||||||||
Beneficial conversion feature of the Series C convertible preferred stock |
|
|
|
|
— |
( |
|
|
|
|
|
|
||||||||||||||||||||
Deemed dividend on the Series C convertible preferred stock |
|
|
|
|
— |
|
|
|
( |
|
|
|
||||||||||||||||||||
Paid-in-kind preferred stock dividend, including beneficial conversion feature |
|
|
|
|
|
|
|
|
— |
|
|
|
|
|
|
( |
|
|
|
|
|
|
||||||||||
Proceeds from exercise of stock options and warrants |
|
|
|
|
|
|
|
|
— |
— |
|
|
|
|
|
|
|
|
|
|
|
|
||||||||||
Balance at December 31, 2020 |
|
|
$ |
|
|
|
$ |
|
|
$ |
|
|
|
$ |
|
$ |
|
$ |
( |
$ |
|
$ |
|
|||||||||
Balance at December 31, 2020 |
|
|
$ |
|
|
|
$ |
|
|
$ |
|
|
|
$ |
|
$ |
|
$ |
( |
$ |
|
$ |
|
|||||||||
Net loss |
|
|
|
|
|
|
|
|
— |
— |
|
|
|
|
|
|
|
( |
|
|
|
( |
||||||||||
Other comprehensive loss, net of taxes |
|
|
|
|
|
|
|
|
— |
— |
|
|
|
|
|
|
|
|
|
( |
|
( |
||||||||||
Stock-based compensation expense |
|
|
|
|
|
|
|
|
— |
— |
|
|
|
|
|
|
|
|
|
|
|
|
||||||||||
Issuance of common stock for board of director compensation |
|
|
|
|
— |
— |
|
|
|
|
|
|
||||||||||||||||||||
Cost of Series C preferred stock conversion |
|
|
|
|
— |
— |
|
|
( |
|
|
( |
||||||||||||||||||||
Issuance of common stock in public offering, net of costs of $ |
|
|
|
|
— |
— |
|
|
|
|
|
|
||||||||||||||||||||
Issuance of common stock in direct placement, net |
|
|
|
|
— |
— |
|
|
|
|
|
|
||||||||||||||||||||
Conversion of Series C preferred shares to common stock |
|
|
|
|
( |
( |
|
|
|
|
|
|
||||||||||||||||||||
Paid-in-kind preferred stock dividend, including beneficial conversion feature |
|
|
|
|
|
|
|
|
— |
|
|
|
|
|
|
( |
|
|
|
|
|
|
||||||||||
Proceeds from exercise of stock options and warrants |
|
|
|
|
|
|
|
— |
— |
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
Balance at December 31, 2021 |
|
$ |
|
|
|
$ |
|
|
$ |
|
|
|
$ |
|
$ |
|
$ |
( |
$ |
( |
$ |
|
||||||||||
See accompanying notes to consolidated financial statements.
F-8
Cryoport, Inc. and Subsidiaries
Consolidated Statements of Cash Flows
(In thousands, except share data)
Years Ended December 31, |
|||||||||
|
2021 |
|
2020 |
|
2019 |
||||
Cash Flows From Operating Activities: |
|
|
|
|
|
|
|||
Net loss |
$ |
( |
$ |
( |
$ |
( |
|||
Adjustments to reconcile net loss to net cash provided by (used in) operating activities: |
|
|
|
|
|
|
|||
Loss on extinguishment of debt |
|
|
|
||||||
Depreciation and amortization |
|
|
|
|
|
|
|||
Amortization of debt discount |
|
|
|
|
|
|
|||
Interest expense on convertible note settled by issuance of common stock |
— |
|
|
||||||
Unrealized (gain) loss on investments in equity securities |
|
|
|
( |
|
|
|||
Realized loss on investments in equity securities |
— |
|
|
||||||
Realized (gain) loss on available-for-sale debt securities |
|
|
( |
||||||
Stock-based compensation expense |
|
|
|
|
|
|
|||
Accelerated stock-based compensation expense |
— |
|
|
||||||
Loss on disposal of property and equipment |
|
|
|
|
|
|
|||
Provision for bad debt |
|
|
|
|
|
|
|||
Changes in operating assets and liabilities, net of effects of acquisition: |
|||||||||
Accounts receivable |
|
( |
|
( |
|
( |
|||
Inventories |
|
( |
|
|
|
( |
|||
Prepaid expenses and other current assets |
|
|
|
( |
|
( |
|||
Deposits |
|
|
|
( |
|
( |
|||
Change in operating lease right-of-use assets and lease liabilities |
|
|
( |
||||||
Accounts payable and other accrued expenses |
|
( |
|
|
|
|
|||
Accrued compensation and related expenses |
|
|
|
|
|
|
|||
Deferred revenue |
|
|
|
( |
|
|
|||
Net deferred tax (asset) liability |
|
( |
|
||||||
Net cash provided by (used in) operating activities |
|
|
|
( |
|
( |
|||
|
|
|
|
||||||
Cash Flows From Investing Activities: |
|
|
|
|
|||||
Purchases of property and equipment |
|
( |
|
( |
|
( |
|||
Purchases of short-term investments |
( |
( |
( |
||||||
Sales/maturities of short-term investments |
|
|
|
|
|
|
|||
Patent and trademark costs |
( |
( |
( |
||||||
Software development costs |
|
( |
|
( |
|
|
|||
Cash paid for acquisitions |
( |
( |
( |
||||||
Net cash used in investing activities |
|
( |
|
( |
|
( |
|||
|
|
|
|
||||||
Cash Flows From Financing Activities: |
|
|
|
|
|||||
Proceeds from exercise of stock options and warrants |
|
|
|
|
|
|
|||
Proceeds from issuance of Series C convertible preferred stock, net of issuance costs |
|
|
|
||||||
Proceeds from issuance of common stock, net of issuance costs |
|
— |
|
|
|
|
|||
Proceeds from public offering, net of offering costs |
|
|
|
||||||
Repayment of finance lease liabilities |
( |
( |
( |
||||||
Repayment of note payable |
( |
|
|
||||||
Proceeds from issuance of convertible senior notes |
|
|
|
||||||
Payment of deferred financing costs |
— |
( |
|
||||||
Net cash provided by financing activities |
|
|
|
|
|
|
|||
|
|
|
|
||||||
Effect of exchange rate changes on cash and cash equivalents |
|
( |
|
|
|
|
|||
Net change in cash and cash equivalents |
|
( |
|
|
|||||
Cash and cash equivalents — beginning of year |
|
|
|
|
|
|
|||
Cash and cash equivalents — end of year |
$ |
|
$ |
|
$ |
|
|||
Supplemental Disclosure of Cash Flow Information: |
|||||||||
Cash paid for interest |
$ |
|
$ |
|
$ |
|
|||
Cash paid for income taxes |
$ |
|
$ |
|
$ |
|
|||
Supplemental Disclosure of Non-Cash Investing and Financing Activities: |
|||||||||
Net unrealized gain (loss) on available-for-sale debt securities |
$ |
|
$ |
|
$ |
( |
|||
Reclassification of realized gain on available-for-sale debt securities to earnings |
$ |
|
$ |
|
$ |
|
|||
Fixed assets included in accounts payable and accrued liabilities |
$ |
|
$ |
|
$ |
|
|||
Purchase of equipment through finance lease obligations |
$ |
— |
$ |
|
$ |
|
|||
Paid-in-kind preferred stock dividend, including beneficial conversion feature |
$ |
|
$ |
|
$ |
|
|||
Common stock issued for conversion of debt and accrued interest |
$ |
|
$ |
|
$ |
|
|||
See accompanying notes to consolidated financial statements.
F-9
Cryoport, Inc. and Subsidiaries
Notes to Consolidated Financial Statements
Note 1. Nature of the Business
Cryoport serves the life sciences industry as a provider of integrated temperature-controlled supply-chain solutions supporting the biopharma/pharma, animal health, and reproductive medicine markets. Our mission is to support life and health worldwide and we are continuously developing, implementing, and leveraging our supply chain platform, which is designed to deliver comprehensive, unparalleled, highly differentiated temperature-controlled logistics, packaging, storage, cryogenic systems, informatics, and related services for life science products, regenerative medicine, cellular therapies, and treatments that require unique, specialized cold chain management.
On October 1, 2020, the Company completed both the MVE Acquisition and the CRYOPDP Acquisition. In addition, in the second quarter of 2021, the Company completed the acquisitions of Critical Transport Solutions Australia (CTSA) in Australia and F-airGate in Belgium to further enhance CRYOPDP’s existing global temperature-controlled supply chain capabilities in the APAC and EMEA regions. These acquisitions are further discussed in Note 3.
The Company is a Nevada corporation and its common stock is traded on the NASDAQ Capital Market exchange under the ticker symbol “CYRX.”
Note 2. Summary of Significant Accounting Policies
Basis of Presentation
The accompanying consolidated financial statements have been prepared in accordance with accounting principles generally accepted in the United States of America (“U.S. GAAP”).
Segment Reporting
The Company continually monitors and reviews its segment reporting structure in accordance with authoritative guidance to determine whether any changes have occurred that would impact its reportable operating segments. Operating segments are defined as components of an enterprise about which separate financial information is available that is evaluated regularly by the chief operating decision maker in deciding how to allocate resources and in assessing operating performance. The chief operating decision maker (“CODM”) is our Chief Executive Officer. Up until the fourth quarter of 2020, we managed, reported and evaluated our business in the following
Principles of Consolidation
The accompanying consolidated financial statements include the accounts of Cryoport, Inc. and its wholly-owned subsidiaries, Cryoport Systems, LLC, Cryogene, Inc., MVE Biological Solutions, US LLC, and Cryoport Netherlands B.V. and subsidiaries, which includes CRYOPDP (collectively, the “Company”). All intercompany accounts and transactions have been eliminated.
Cash and Cash Equivalents
Our cash and cash equivalents represent demand deposits, and money market funds which are readily convertible into cash, have maturities of
F-10
Short-Term Investments
Our investments in equity securities consist of mutual funds with readily determinable fair values which are carried at fair value with changes in fair value recognized in earnings.
Investments in debt securities are classified as available-for-sale and are carried at fair value, with unrealized gains and losses, net of tax, reported as accumulated other comprehensive income (loss) and included as a separate component of stockholders’ equity.
Gains and losses are recognized when realized. When we have determined that an other than temporary decline in fair value has occurred, the amount related to a credit loss is recognized in earnings. Gains and losses are determined using the specific identification method.
Short-term investments are classified as current assets even though maturities may extend beyond one year because they represent investments of cash available for operations.
Use of Estimates
The preparation of the consolidated financial statements in conformity with U.S. GAAP requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities and disclosure of contingent assets and liabilities at the date of the consolidated financial statements and the reported amounts of revenues and expenses during the reporting periods. Actual results could differ from estimated amounts. The Company’s significant estimates include the allowance for doubtful accounts, fair value of short-term investments, valuations and purchase price allocations related to business combinations, expected future cash flows including growth rates, discount rates, terminal values and other assumptions and estimates used to evaluate the recoverability of long-lived assets, estimated fair values of intangible assets and goodwill, intangible asset useful lives and amortization methods, allowance for inventory obsolescence, equity-based instruments, tax reserves and recoverability of the Company’s net deferred tax assets and related valuation allowance.
Although the Company regularly assesses these estimates, actual results could differ materially from these estimates. Changes in estimates are recorded in the period in which they become known. The Company bases its estimates on historical experience and various other assumptions that it believes to be reasonable under the circumstances.
Future events, including the extent and the duration of the COVID-19 related economic impacts, and their effects cannot be predicted with certainty, and, accordingly the Company’s accounting estimates require the exercise of judgment.
Fair Value of Financial Instruments
The Company’s financial instruments consist of cash and cash equivalents, short-term investments, accounts receivable, accounts payable and accrued expenses, finance lease liabilities, note payable, and the Company’s convertible senior notes due in 2025 and 2026 (the “Senior Notes”). The carrying value for all such instruments, except finance lease liabilities, note payable and the Senior Notes, approximates fair value at December 30, 2021 and December 31, 2020 due to their short-term nature. The carrying value of finance lease liabilities approximates fair value because the interest rate approximates market rates available to us for similar obligations with the same maturities. For additional information related to fair value measurements, including the note payable and the Senior Notes, see Note 10.
Concentrations of Credit Risk
Financial instruments that potentially subject us to concentrations of credit risk consist primarily of cash, cash equivalents and short-term investments. From time to time, we maintain cash, cash equivalent and short-term investment balances in excess of amounts insured by the Federal Deposit Insurance Corporation (“FDIC”) and the Securities Investor Protection Corporation (“SIPC”). Primarily all of our cash, cash equivalents and short-term investments at December 31, 2021 were in excess of amounts insured by the FDIC and SIPC. The Company performs ongoing evaluations of these institutions to limit its concentration risk exposure. We manage such risks in our portfolio by investing in highly liquid, highly rated instruments, and limit investing in long-term maturity instruments.
Our investment policy requires that purchased instruments in marketable securities may only be in highly rated instruments, which are primarily U.S. Treasury bills or treasury-backed securities, and also limits our investment in securities of any single issuer.
F-11
Customers
The Company grants credit to customers within the U.S. and to international customers and does not require collateral. The Company generally requires advance or credit card payments for initial revenues from new customers. The Company’s ability to collect receivables is affected by economic fluctuations in the geographic areas and industries served by the Company. Reserves for uncollectible amounts are provided based on past experience and a specific analysis of the accounts, which management believes is sufficient. Accounts receivable at December 31, 2021, and 2020 are net of reserves for doubtful accounts of $
The Company’s customers are in the biotechnology, pharmaceutical, animal health, human reproductive medicine and other life science industries. Consequently, there is a concentration of accounts receivable within these industries, which is subject to normal credit risk. As of December 31, 2021, there was no single customer that owed us more than
The Company has revenue from foreign customers primarily in the United Kingdom, France, Germany, China and India. During the years ended December 31, 2021, 2020 and 2019, the Company had revenues from foreign customers of approximately $
Inventories
Inventories are stated at the lower of cost and net realizable value. Cost is determined using the first-in, first-out (“FIFO”) method. Inventories are reviewed periodically for slow-moving or obsolete status. The Company writes down the carrying value of its inventories to reflect situations in which the cost of inventories is not expected to be recovered. Once established, write-downs of inventories are considered permanent adjustments to the cost basis of the obsolete or excess inventories. Raw materials and finished goods include material costs less reserves for obsolete or excess inventories. The Company evaluates the current level of inventories considering historical trends and other factors, such as selling prices and costs of completion, disposal and transportation, and based on the evaluation, records adjustments to reflect inventories at net realizable value. These adjustments are estimates, which could vary significantly from actual results if future economic conditions, customer demand, competition or other relevant factors differ from expectations. These estimates require us to make assessments about future demand for the Company’s products in order to categorize the status of such inventories items as slow-moving, obsolete or in excess-of-need. These estimates are subject to the ongoing accuracy of the Company’s forecasts of market conditions, industry trends, competition and other factors.
Property and Equipment
Property and equipment are stated at cost, less accumulated depreciation. We compute depreciation using the straight-line method over the estimated useful lives of the assets which is generally to
Betterments, renewals and extraordinary repairs that extend the lives of the assets are capitalized; other repairs and maintenance charges are expensed as incurred. The cost and related accumulated depreciation and amortization applicable to assets retired are removed from the accounts, and the gain or loss on disposition is recognized in the consolidated statements of operations.
Leases
The Company determines if an arrangement is a lease at inception. Operating lease right-of-use (“ROU”) assets represent the Company’s right to use an underlying asset during the lease term, and operating lease liabilities represent the Company’s obligation to make lease payments arising from the lease. Operating leases are included in ROU assets, current operating lease liabilities, and long-term operating lease liabilities on our consolidated balance sheets. Finance leases are included in property and equipment, current finance lease liabilities, and long-term finance lease liabilities on our consolidated balance sheets.
F-12
Lease ROU assets and lease liabilities are initially recognized based on the present value of the future minimum lease payments over the lease term at commencement date calculated using our incremental borrowing rate applicable to the lease asset, unless the implicit rate is readily determinable. ROU assets also include any lease payments made at or before lease commencement and exclude any lease incentives received. Our lease terms may include options to extend or terminate the lease when it is reasonably certain that we will exercise that option. Leases with a term of 12 months or less are not recognized on the consolidated balance sheets. The Company’s leases do not contain any residual value guarantees. Lease expense for minimum lease payments is recognized on a straight-line basis over the lease term.
The Company accounts for lease and non-lease components as a single lease component for all its leases.
Business Combinations
Total consideration transferred for acquisitions is allocated to the assets acquired and liabilities assumed based on their fair values at the dates of acquisition. This purchase price allocation process requires management to make significant estimates and assumptions primarily with respect to intangible assets. The fair value of identifiable intangible assets is based on detailed valuations that use information and assumptions determined by management. Any excess of purchase price over the fair value of the net tangible and intangible assets acquired is allocated to goodwill. While the Company uses its best estimates and assumptions to accurately value assets acquired and liabilities assumed at the acquisition date as well as any contingent consideration, where applicable, the Company’s estimates are inherently uncertain and subject to refinement. As a result, during the measurement period, which may be up to one year from the acquisition date, the Company records adjustments to the assets acquired and liabilities assumed with the corresponding offset to goodwill.
Goodwill
The Company evaluates goodwill on an annual basis in the fourth quarter or more frequently if management believes indicators of impairment exist. Such indicators could include, but are not limited to: (1) a significant adverse change in legal factors or in business climate, (2) unanticipated competition, or (3) an adverse action or assessment by a regulator. The Company compares the fair value of the reporting unit with its carrying amount and then recognizes an impairment charge for the amount by which the carrying amount exceeds the reporting unit’s fair value up to the total amount of goodwill allocated to the reporting unit. The Company assessed triggering events indicating potential goodwill impairment, including the effects of the COVID-19 pandemic, and after assessment, concluded that there was
Intangible Assets
Intangible assets are comprised of patents, trademarks, software development costs and the intangible assets acquired in the Company’s recent acquisitions which include a non-compete agreement, technology, customer relationships, trade name/trademark, agent network, order backlog, developed technology and land use rights. These intangible assets are amortized using the straight-line method over the estimated useful lives (see Note 8). The Company uses the following valuation methodologies to value the significant intangible assets acquired: income approach for customer relationships, replacement cost for agent network and software, and relief from royalty for trade name/trademarks and developed technology . The Company capitalizes costs of obtaining patents and trademarks, which are amortized, using the straight-line method over their estimated useful life of
F-13
The Company evaluates the recoverability of identifiable intangible assets whenever events or changes in circumstances indicate that an intangible asset's carrying amount may not be recoverable. Such circumstances could include, but are not limited to: (1) a significant decrease in the market value of an asset, (2) a significant adverse change in the extent or manner in which an asset is used, or (3) an accumulation of costs significantly in excess of the amount originally expected for the acquisition of an asset. The Company measures the carrying amount of the asset against the estimated undiscounted future cash flows associated with it. Should the sum of the expected future net cash flows be less than the carrying value of the asset being evaluated, an impairment loss would be recognized. The impairment loss would be calculated as the amount by which the carrying value of the asset exceeds its fair value. The estimate of fair value is based on various valuation techniques, including the discounted value of estimated future cash flows. The evaluation of asset impairment requires the Company to make assumptions about future cash flows over the life of the asset being evaluated. These assumptions require significant judgment and actual results may differ from assumed and estimated amounts. There was
Other Long-lived Assets
If indicators of impairment exist, we assess the recoverability of the affected long-lived assets by determining whether the carrying value of such assets can be recovered through undiscounted future operating cash flows. If impairment is indicated, we measure the amount of such impairment by comparing the fair value to the carrying value. We believe the future cash flows to be received from the long-lived assets will exceed the assets’ carrying value, and accordingly, we have not recognized any impairment losses through December 31, 2021.
Deferred Financing Costs
Deferred financing costs represent costs incurred in connection with the issuance of debt instruments and equity financings. Deferred financing costs related to the issuance of debt are amortized over the term of the financing instrument using the effective interest method and are presented in the consolidated balance sheets as an offset against the related debt. Offering costs from equity financings are netted against the gross proceeds received from the equity financings.
Income Taxes
The Company accounts for income taxes under the provision of Accounting Standards Codification (“ASC”) 740, “Income Taxes”, or ASC 740. As of December 31, 2021,2020 and 2019, there were
Deferred tax assets and liabilities are measured using enacted tax rates expected to apply to taxable income in the years in which those temporary differences are expected to be recovered or settled. The effect on deferred tax assets and liabilities of a change in tax rates is recognized in income in the period that includes the enactment date. A valuation allowance is provided for deferred tax assets if it is more likely than not that the Company will not realize the deferred tax assets through future operations. Based on the weight of available evidence, the Company’s management has determined that it is not more likely than not that the U.S. based net deferred tax assets will be realized. Therefore, the Company has recorded a full valuation allowance against its U.S. based net deferred tax assets. With respect to foreign based deferred tax assets, the Company's management has reviewed these deferred tax assets on a jurisdictional basis. Based on the weight of each jurisdiction's available evidence, the Company's management has made separate determinations for each foreign jurisdiction regarding whether it is more likely than not that a net deferred tax asset within a particular jurisdiction will be realized. The Company has recorded full valuation allowances in jurisdictions where deferred tax assets are not deemed more likely than not to be realized.
The Company has recorded a net deferred tax liability in juristictions where taxable termporary differences associated with indefinite-lived intangible assets do not support the realization of deferred tax assets with finite carryforward periods. In addition, the Company has recorded a net deferred tax liability in juristictions where taxable temporary differences exceed deductible temporary differences.
The Company’s policy is to recognize interest and/or penalties related to income tax matters in income tax expense. The Company has recorded immaterial accruals for interest and/or penalties on its consolidated balance sheets at December 31, 2021, and 2020, and has recorded immaterial amounts of interest and/or penalties in the consolidated statements of operations for the years ended December 31, 2021 and 2020. The Company is subject to taxation in the U.S., in various U.S. state jurisdictions and in various foreign countries. As of December 31, 2021, the Company is no longer subject to U.S. federal examinations for years before 2018 or for
F-14
California franchise and income tax examinations for years before 2017. However, to the extent allowed by law, the taxing authorities may have the right to examine net operating losses carried forward into a tax year and make adjustments up to the amount of the net operating losses utilized. The Company is not currently under examination in either the U.S. federal or any U.S. state jurisdictions. Our foreign subsidiaries are generally subject to examination for three years following the year in which the tax obligation originated. The years subject to audit may be extended if the entity substantially understates corporate income tax. The Company's subsidiary in India is currently under examination by the Indian tax authorities for the 2012-2013, 2013-2014 and 2015-2016 tax periods. Other than India, the Company does not have any foreign subsidiaries currently under audit by their local taxing authorities.
On March 27, 2020, the United States enacted the Coronavirus Aid, Relief and Economic Security Act (CARES Act). The CARES Act is an emergency economic stimulus package that includes spending and tax breaks to strengthen the United States economy and fund a nationwide effort to curtail the effect of COVID-19. The CARES Act provides sweeping tax changes in response to the COVID-19 pandemic. Some of the more significant provisions include the removal of certain limitations on utilization of net operating losses, increasing the loss carryback period for certain losses to five years, and increasing the ability to deduct interest expense, as well as amending certain provisions of the previously enacted Tax Cuts and Jobs Act. As of December 31, 2021, the Company has not recorded any income tax provision/(benefit) resulting from the CARES Act mainly due the Company’s history of net operating losses generated and the maintenance of a full valuation allowance against its net deferred tax assets.
On June 29, 2020, the State of California passed Assembly Bill 85 which suspends the California net operating loss deduction for the 2020-2022 tax years and the R&D credit usage for the same period (for credit usages in excess of $
On December 27, 2020, the United States enacted the Consolidated Appropriations Act of 2021 (“CAA”). The CAA includes provisions extending certain CARES Act provisions and adds coronavirus relief, tax and health extenders. The Company has evaluated the impact of the CAA and considered any potential impact on our financial statements in 2021 and beyond.
On March 11, 2021, the United States enacted the American Rescue Plan (“ARP”). The ARP includes provisions extending certain CARES Act provisions, repeals a worldwide interest allocation election, modifies the $
Revenue Recognition
Revenues are recognized when control is transferred to customers, in an amount that reflects the consideration the Company expects to be entitled to in exchange for those goods and services. Revenue recognition is evaluated through the following five steps: (i) identification of the contract, or contracts, with a customer; (ii) identification of the performance obligations in the contract; (iii) determination of the transaction price; (iv) allocation of the transaction price to the performance obligations in the contract; and (v) recognition of revenue when or as a performance obligation is satisfied.
Performance Obligations
At contract inception, an assessment of the goods and services promised in the contracts with customers is performed and a performance obligation is identified for each distinct promise to transfer to the customer a good or service (or bundle of goods or services). To identify the performance obligations, the Company considers all of the goods or services promised in the contract regardless of whether they are explicitly stated or are implied by customary business practices. Revenue is recognized when our performance obligation has been met. The Company considers control to have transferred upon delivery because the Company has a present right to payment at that time, the Company has transferred use of the asset, and the customer is able to direct the use of, and obtain substantially all of the remaining benefits from, the asset.
For arrangements under which the Company provides biological specimen storage services and logistics support and management to the customer, the Company satisfies its performance obligations as those services are performed whereby the customer simultaneously receives and consumes the benefits of such services under the agreement.
Revenue generated from short-term logistics and engineering consulting services provided to customers is recognized when the Company satisfies the contractually defined performance obligations. When a contract includes multiple performance obligations, the contract price is allocated among the performance obligations based upon the stand-alone selling prices. Approved contract
F-15
modifications are accounted for as either a separate contract or as part of the existing contract depending on the nature of the modification.
Our performance obligations on our orders and under the terms of agreements with customers are generally satisfied within one year from a given reporting date and, therefore,
Shipping and handling activities related to contracts with customers are accounted for as costs to fulfill our promise to transfer the associated products pursuant to the accounting policy election allowed under Topic 606 and are not considered a separate performance obligation to our customers. Accordingly, the Company records amounts billed for shipping and handling as a component of revenue. Shipping and handling fees and costs are included in cost of revenues in the accompanying consolidated statements of operations.
Revenues are recognized net of any taxes collected from customers, which are subsequently remitted to governmental agencies.
Significant Payment Terms
Pursuant to the Company’s contracts with its customers, amounts billed for services or products delivered by the Company are generally due and payable in full within 15 to 60 days from the date of the invoice (except for any amounts disputed by the customer in good faith). Accordingly, the Company determined that its contracts with customers do not include extended payment terms or a significant financing component.
Variable Consideration
When a contract includes variable consideration, the Company evaluates the estimate of the variable consideration to determine whether the estimate needs to be constrained. Variable consideration is estimated at the most likely amount that is expected to be earned. Estimated amounts are included in the transaction price to the extent it is probable that a significant reversal of cumulative revenue recognized will not occur when the uncertainty associated with the variable consideration is resolved. Estimates of variable consideration and determination of whether to include estimated amounts in the transaction price are based largely on an assessment of the anticipated performance and all information (historical, current and forecasted) that is reasonably available. Variable consideration estimates are updated at each reporting date. Revenues are recorded net of variable consideration, such as discounts and allowances.
Warranties
The Company provides product warranties with varying terms and durations for some of its products. The Company estimates product warranty costs and accrues for these costs as products are sold with a charge to cost of sales. Factors considered in estimating warranty costs include historical and projected warranty claims, historical and projected cost-per-claim, and knowledge of specific product issues that are outside of typical experience. Warranty accruals are evaluated and adjusted as necessary based on actual claims experience and changes in future claim and cost estimates.
Product warranty accrued liabilities totaled $
Incremental Direct Costs
Incremental direct costs of obtaining a contract (sales commissions) are expensed when incurred when the amortization period of the asset that would have been recognized is one year or less; otherwise, incremental contract costs are recognized as an asset and amortized over time as promised goods and services are transferred to a customer. Incremental direct costs were not material for the years ended December 31, 2021, 2020 and 2019.
Contract Assets
Typically, we invoice the customer and recognize revenue once we have satisfied our performance obligation. Accordingly, our contract assets comprise accounts receivable, which are recognized when payment is unconditional and only the passage of time is
F-16
required before payment is due. Generally, we do not have material amounts of other contract assets since revenue is recognized as control of goods is transferred or as services are performed.
Contract Liabilities (Deferred Revenue)
Contract liabilities are recorded when cash payments are received in advance of the Company’s performance. Deferred revenue was $
Nature of Goods and Services
The Company provides Cryoport Express® Shippers to its customers and charges a fee in exchange for the use of the Cryoport Express® Shipper under long-term master service agreements with customers. The Company’s arrangements convey to the customers the right to use the Cryoport Express® Shippers over a period of time. The Company retains title to the Cryoport Express® Shippers and provides its customers the use of the Cryoport Express® Shipper for a specified shipping cycle. At the culmination of the customer’s shipping cycle, the Cryoport Express® Shipper is returned to the Company.
The Company recognizes revenue for the use of the Cryoport Express® Shippers at the time of the delivery of the Cryoport Express® Shipper to the end user of the enclosed materials, and at the time that collectability is probable.
The Company also provides vacuum insulated aluminum dewars and cryogenic freezers systems to its customers. Revenue is recognized when the Company satisfies performance obligations by transferring the equipment to a customer, and at the time that collectability is probable.
The Company also provides global temperature-controlled logistics services, support and management. Revenue is recognized for these services as services are rendered and at the time that collectability is probable.
The Company also provides comprehensive and integrated temperature-controlled biostorage solutions to customers in the life sciences industry and charges a fee under long-term master service agreements with customers. These services include (1) biological specimen cryopreservation storage and maintenance, (2) archiving, monitoring, tracking, receipt and delivery of samples, (3) transport of frozen biological specimens to and from customer locations, and (4) management of incoming and outgoing biological specimens. The Company recognizes revenue for its biostorage solutions as services are rendered over time and at the time that collectability is probable.
The Company also provides short-term logistics and engineering consulting services to some customers, with fees tied to the completion of contractually defined services. We recognize revenue from these services over time as the customer simultaneously receives and consumes the benefit of these services as they are performed.
A significant portion of our revenues are covered under long-term agreements. We have determined that individual Statements of Work or Scope of Work (“SOW”), whose terms and conditions taken with a Master Services Agreement (“MSA”), create the Topic 606 contracts which are generally short-term in nature (e.g., 15-day shipping cycle) for the Cryoport Express® solutions and up to 12 months for biostorage solutions. Our agreements (including SOWs) generally do not have multiple performance obligations and, therefore, do not require an allocation of a single price amongst multiple goods or services. Prices under these agreements are generally fixed.
F-17
Revenue Disaggregation
The Company views its operations, makes decisions regarding how to allocate resources and manages its business as
December 31, |
|||||||||
|
2021 |
|
2020 |
|
2019 |
||||
Biopharma/Pharma |
$ |
|
$ |
|
$ |
|
|||
Animal Health |
|
|
|
|
|
|
|||
Reproductive Medicine |
|
|
|
||||||
Total Revenues |
$ |
|
$ |
|
$ |
|
|||
Given that the Company’s revenues are generated in different geographic regions, factors such as regulatory and geopolitical factors within those regions could impact the nature, timing and uncertainty of the Company’s revenues and cash flows. Our geographical revenues, by origin, for the years ended December 31, 2021, 2020 and 2019,were as follows (in thousands):
December 31, |
|||||||||
|
2021 |
|
2020 |
|
2019 |
||||
Americas |
$ |
|
$ |
|
$ |
|
|||
Europe, the Middle East and Africa (EMEA) |
|
|
|
|
|
|
|||
Asia Pacific (APAC) |
|
|
|
|
|
|
|||
Total revenues |
$ |
|
$ |
|
$ |
|
|||
Cost of Service Revenues
Our cost of service revenues is primarily comprised of freight charges, payroll and associated expenses related to our global logistics and supply chain centers, depreciation expenses of our Cryoport Express® Shippers and supplies and consumables used for our solutions.
Cost of Product Revenues
Our cost of product revenues is primarily comprised of materials, direct and indirect labor, inbound freight charges, purchasing and receiving, inspection, and distribution and warehousing of inventory. In addition, shop supplies, facility maintenance costs and depreciation expense for assets used in the manufacturing process are included in cost of product revenues.
Engineering and Development Expenses
Expenditures relating to engineering and development are expensed in the period incurred to engineering and development expense in the consolidated statements of operations.
Acquisition Costs
Acquisition costs consist of legal, accounting, third-party valuations, and other due diligence costs related to our acquisitions.
F-18
Stock-Based Compensation
Under our stockholder approved stock-based compensation plan, we have granted incentive stock options, non-qualified stock options and restricted stock units that vest over
The grant date fair value per share for restricted stock units is based upon the closing market price of our common stock on the award grant date.
The Company’s stock-based compensation plans are discussed further in Note 16.
Equity Instruments Issued to Non-Employees for Acquiring Goods or Services
Issuances of the Company’s common stock for acquiring goods or services are measured at the estimated fair value of the consideration received or the estimated fair value of the equity instruments issued, whichever is more reliably measurable. The measurement date for the estimated fair value of the equity instruments issued to consultants or vendors is determined at the earlier of (i) the date at which a commitment for performance to earn the equity instruments is reached (a “performance commitment” which would include a penalty considered to be of a magnitude that is a sufficiently large disincentive for nonperformance) or (ii) the date at which performance is complete. When it is appropriate for the Company to recognize the cost of a transaction during financial reporting periods prior to the measurement date, for purposes of recognition of costs during those periods, the equity instrument is measured at the then-current estimated fair values.
Basic and Diluted Net Loss Per Share
We calculate basic and diluted net loss per share using the weighted average number of common shares outstanding during the periods presented. In periods of a net loss position, basic and diluted weighted average common shares are the same. For the diluted earnings per share calculation, we adjust the weighted average number of common shares outstanding to include dilutive stock options, warrants, unvested restricted stock units and shares associated with the conversion of the Convertible Senior Notes and convertible preferred stock outstanding during the periods. We use the if-converted method for calculating any potential dilutive effect of the Convertible Senior Notes and convertible preferred stock on diluted net loss per share.
The following shows the amounts used in computing net loss per share (in thousands except per share data):
Years Ended December 31, |
|||||||||
|
2021 |
|
2020 |
|
2019 |
||||
Net loss |
$ |
( |
$ |
( |
$ |
( |
|||
Deemed dividend on Series C convertible preferred stock |
— |
( |
— |
||||||
Paid-in-kind dividend on Series C convertible preferred stock |
|
( |
|
( |
|
— |
|||
Net loss attributable to common shareholders |
$ |
( |
$ |
( |
$ |
( |
|||
Weighted average common shares outstanding - basic and diluted |
|
|
|
||||||
Net loss per share attributable to common stockholders – basic and diluted |
( |
( |
( |
||||||
F-19
The following table sets forth the number of shares excluded from the computation of diluted loss per share, as their inclusion would have been anti-dilutive:
Years Ended December 31, |
||||||
|
2021 |
|
2020 |
|
2019 |
|
Stock options |
|
|
|
|
|
|
Restricted stock units |
|
— |
— |
|||
Warrants |
— |
— |
|
|||
Series C convertible preferred stock |
|
|
— |
|||
Convertible Senior Notes |
|
|
|
|
— |
|
|
|
|
|
|
||
Foreign Currency Transactions
Management has determined that the functional currency of its subsidiaries is the local currency. The Company translates the assets and liabilities of its foreign subsidiaries into U.S. dollars at exchange rates in effect at the end of the reporting period. Income and expenses are translated at an average exchange rate for the period and the resulting translation gain (loss) adjustments are accumulated as a separate component of stockholders’ equity. The translation gain (loss) adjustment totaled ($
Off Balance Sheet Arrangements
We do not currently have any off balance sheet arrangements.
Reclassification
Prior year amounts in sales and marketing expense have been reclassified to selling, general and administrative expense to conform to the current period presentation. These reclassifications had no effect on the previously reported net loss.
Recently Adopted Accounting Pronouncements
In August 2020, the FASB issued ASU 2020-06, “Debt—Debt with Conversion and Other Options (Subtopic 470-20) and Derivatives and Hedging—Contracts in Entity’s Own Equity (Subtopic 815-40): Accounting for Convertible Instruments and Contracts in an Entity’s Own Equity”. Under ASU 2020-06, the embedded conversion features are no longer separated from the host contract for convertible instruments with conversion features that are not required to be accounted for as derivatives under Topic 815, Derivatives and Hedging, or that do not result in substantial premiums accounted for as paid-in capital. Consequently, a convertible debt instrument will be accounted for as a single liability measured at its amortized cost, as long as no other features require bifurcation and recognition as derivatives. Similarly, equity-classified convertible preferred stock instruments will be accounted for as single units of account in equity unless the conversion feature needs to be bifurcated under Topic 815. The new guidance also made amendments to the earnings per share guidance in Topic 260, Earnings Per Share, for convertible instruments, the most significant impact of which is requiring the use of the if-converted method for diluted earnings per share calculation. Further, ASU 2020-06 made revisions to Topic 815-40, which provides guidance on how an entity must determine whether a contract qualifies for a scope exception from derivative accounting. ASU 2020-06 is effective for fiscal years beginning after December 15, 2021, with early adoption permitted. Adoption of the standard requires using either a modified retrospective or a full retrospective approach. Effective January 1, 2021, we early adopted ASU 2020-06 using the modified retrospective approach. Adoption of the new standard did not have a material impact on the Company’s consolidated financial statements or disclosures.
In January 2020, the FASB issued ASU 2020-01, “Investments—Equity Securities (Topic 321), Investments—Equity Method and Joint Ventures (Topic 323), and Derivatives and Hedging (Topic 815): Clarifying the Interactions between Topic 321, Topic 323, and Topic 815.” The new guidance clarifies the interaction of accounting for the transition into and out of the equity method and the accounting for measuring certain purchased options and forward contracts to acquire investments. ASU 2020-01 is effective for fiscal years beginning after December 15, 2020, including interim periods within those fiscal years. We adopted this guidance on January 1, 2021. The adoption of this guidance did not have an impact on the Company’s consolidated financial statements or disclosures.
F-20
Accounting Guidance Issued but Not Adopted at December 31, 2021
In October 2021, the FASB issued ASU 2021-08, “Business Combinations (Topic 805): Accounting for Contract Assets and Contract Liabilities from Contracts with Customers.” ASU 2021-08 requires contract assets and contract liabilities acquired in a business combination to be recognized and measured in accordance with Topic 606, Revenue from Contracts with Customers, on the acquisition date as if the acquirer had entered into the original contract at the same date and on the same terms as the acquiree. ASU 2021-08 is effective for fiscal years beginning after December 15, 2022, including interim periods within those fiscal years for public business entities. We are currently evaluating the impact of this standard on our consolidated financial statements.
In July 2021, the FASB issued ASU 2021-05, “Leases (Topic 842): Lessors—Certain Leases with Variable Lease Payments.” Under this ASU, lessors should classify and account for a lease with variable lease payments that do not depend on a reference index or a rate as an operating lease if both of the following criteria are met: (1) the lease would have been classified as a sales-type lease or a direct financing lease in accordance with the classification criteria in Topic 842 and (2) the lessor would have otherwise recognized a day-one loss. ASU 2021-05 is effective for fiscal years beginning after December 15, 2021 and interim periods within those fiscal years for all public business entities. We are currently evaluating the impact of this standard on our consolidated financial statements.
In May 2021, the FASB issued ASU 2021-04, “Earnings Per Share (Topic 260), Debt — Modifications and Extinguishments (Subtopic 470-50), Compensation — Stock Compensation (Topic 718), and Derivatives and Hedging — Contracts in Entity's Own Equity (Subtopic 815-40): Issuer's Accounting for Certain Modifications or Exchanges of Freestanding Equity-Classified Written Call Options (a consensus of the Emerging Issues Task Force).” ASU 2021-04 requires issuers to account for modifications or exchanges of freestanding equity-classified written call options that remain equity classified after the modification or exchange based on the economic substance of the modification or exchange. Under the guidance, an issuer determines the accounting for the modification or exchange based on whether the transaction was done to issue equity, to issue or modify debt, or for other reasons. ASU 2021-04 is applied prospectively and is effective for fiscal years beginning after December 15, 2021, and interim periods within those fiscal years. We are currently evaluating the impact of this standard on our consolidated financial statements.
In June 2016, the FASB issued ASU 2016-13, "Measurement of Credit Losses on Financial Instruments." This ASU replaces the incurred loss impairment methodology in current U.S. GAAP with a methodology that reflects expected credit losses and requires consideration of a broader range of reasonable and supportable information for credit loss estimates on certain types of financial instruments, including trade receivables. In addition, new disclosures are required. The ASU, as subsequently amended, is effective for the Company for fiscal years beginning after December 15, 2022 as the Company was a smaller reporting company as of November 15, 2019, the determination date. We are currently evaluating the impact of adopting this guidance.
Note 3. Acquisitions
2021 Acquisitions
In the second quarter of 2021, we completed the acquisitions of Critical Transport Solutions Australia (CTSA) in Australia and F-airGate in Belgium to further enhance our existing global temperature-controlled supply chain capabilities in the APAC and EMEA regions. The combined purchase consideration was $
F-21
2020 Acquisitions
CRYOPDP Acquisition
On October 1, 2020, the Company completed its acquisition of CRYOPDP for a cash consideration of €
The CRYOPDP Acquisition was accounted for under the acquisition method of accounting in accordance with FASB ASC Topic 805, “Business Combinations,” and, therefore, the total purchase price was allocated to the identifiable tangible and intangible assets acquired and the liabilities assumed based on their respective fair values on the acquisition date. Fair values were determined by management based in part on an independent valuation performed by a third-party valuation specialist and required the use of significant assumptions and estimates. Critical estimates included, but were not limited to, future expected cash flows, including projected revenues and expenses, and the applicable discount rates. These estimates were based on assumptions that the Company believes to be reasonable; however, actual results may differ from these estimates.
The following table summarizes the allocation of the purchase price as of the acquisition date (in thousands):
Total purchase consideration paid |
|
$ |
|
Purchase price allocation: |
|
|
|
Cash and cash equivalents |
|
|
|
Accounts receivable |
|
|
|
Inventories |
|
|
|
Prepaid and other current assets |
|
|
|
Property and equipment |
|
|
|
Operating lease right-of-use assets |
|
|
|
Intangible assets |
|
|
|
Other long-term assets |
|
|
|
Accounts payable and other accrued expenses |
|
( |
|
Accrued compensation and related expenses |
|
( |
|
Deferred revenue |
|
( |
|
Note payable |
|
( |
|
Operating lease liabilities |
|
( |
|
Deferred tax liability |
|
( |
|
Other long-term liabilities |
|
( |
|
Total identifiable net assets |
|
|
|
Goodwill |
|
|
|
$ |
|
The following table summarizes the estimated fair values of CRYOPDP’s identifiable intangible assets at the date of acquisition and their estimated useful lives and amortization expense based on their respective useful lives (in thousands):
Annual |
||||||||||
Estimated |
Estimated |
Amortization |
Amortization |
|||||||
|
Fair Value |
|
Useful Life |
|
Method |
|
Expense |
|||
Software |
$ |
|
|
|
Straight-line |
$ |
|
|||
Customer relationships |
|
|
|
|
Straight-line |
|
|
|||
Agent network |
|
|
|
|
Straight-line |
|
|
|||
Trade name/trademarks |
|
|
|
Indefinite |
|
— |
|
— |
||
Total |
$ |
|
|
|
$ |
|
||||
F-22
Goodwill is calculated as the excess of the purchase price over the fair value of net assets acquired and represents the future economic benefits arising from other assets acquired that could not be individually identified and separately recognized. Among the factors that contributed to a purchase price in excess of the fair value of the net tangible and intangible assets acquired were the acquisition of an assembled workforce, the expected synergies, and other benefits that we believe will result from combining the operations of CRYOPDP with our operations. The goodwill recognized of $
Acquisition-related transaction costs (included in selling, general and administrative expenses) totaled approximately $
The final purchase price for the CRYOPDP Acquisition was $
MVE Acquisition
On October 1, 2020, the Company completed its acquisition of Chart Industries, Inc.’s MVE cryobiological storage business for a cash consideration of $
The MVE Acquisition was accounted for under the acquisition method of accounting in accordance with FASB ASC Topic 805, “Business Combinations,” and, therefore, the total purchase price was allocated to the identifiable tangible and intangible assets acquired and the liabilities assumed based on their respective fair values on the acquisition date. Fair values were determined by management based in part on an independent valuation performed by a third-party valuation specialist and required the use of significant assumptions and estimates. Critical estimates included, but were not limited to, future expected cash flows, including projected revenues and expenses, and the applicable discount rates. These estimates were based on assumptions that the Company believes to be reasonable; however, actual results may differ from these estimates.
F-23
The following table summarizes the allocation of the purchase price as of the acquisition date (in thousands):
Total purchase consideration paid |
|
$ |
|
Purchase price allocation: |
|
|
|
Cash and cash equivalents |
|
|
|
Accounts receivable |
|
|
|
Inventories |
|
|
|
Other current assets |
|
|
|
Property and equipment |
|
|
|
Operating lease right-of-use assets |
|
|
|
Intangible assets |
|
|
|
Other non-current assets |
|
|
|
Accounts payable and other accrued expenses |
|
( |
|
Accrued compensation and related expenses |
|
( |
|
Operating lease liabilities |
|
( |
|
Deferred tax liabilities |
|
( |
|
Other long-term liabilities |
|
( |
|
Total identifiable net assets |
|
|
|
Goodwill |
|
|
|
$ |
|
The following table summarizes the estimated fair values of MVE’s identifiable intangible assets and their estimated useful lives and amortization expense based on their respective useful lives (in thousands):
Annual |
||||||||||
Estimated |
Estimated |
Amortization |
Amortization |
|||||||
|
Fair Value |
|
Useful Life |
|
Method |
|
Expense |
|||
Order backlog |
|
|
|
|
Straight-line |
$ |
— |
|||
Customer relationships |
|
|
|
|
Straight-line |
|
|
|||
Developed technology |
|
|
|
|
Straight-line |
|
|
|||
Land use rights |
|
|
|
|
Straight-line |
|
|
|||
Trade name/trademarks |
|
|
|
Indefinite |
|
— |
|
— |
||
Total |
$ |
|
|
|
$ |
|
||||
Goodwill is calculated as the excess of the purchase price over the fair value of net assets acquired and represents the future economic benefits arising from other assets acquired that could not be individually identified and separately recognized. Among the factors that contributed to a purchase price in excess of the fair value of the net tangible and intangible assets acquired were the acquisition of an assembled workforce, the expected synergies, and other benefits that we believe will result from combining the operations of MVE with our operations. Of the $
Acquisition-related transaction costs (included in selling, general and administrative expenses) totaled approximately $
The final purchase price for the MVE Acquisition was $
F-24
Note 4. Cash, Cash Equivalents and Short-term Investments
Cash, cash equivalents and short-term investments consisted of the following as of December 31, 2021 and 2020 (in thousands):
Carrying Value |
||||||
|
2021 |
|
2020 |
|||
Cash |
$ |
|
|
|||
Cash equivalents: |
|
|||||
Money market mutual funds |
|
|
|
|||
Total cash and cash equivalents |
|
|
|
|||
Short-term investments: |
|
|||||
U.S. Treasury notes and bills |
|
|
|
|||
Mutual funds |
|
|
|
|||
Corporate debt securities |
|
— |
||||
Total short-term investments |
|
|
|
|||
Cash, cash equivalents and short-term investments |
$ |
|
$ |
|
||
Available-for-sale debt securities
The amortized cost, gross unrealized gains, gross unrealized losses and fair value of available-for-sale debt securities by type of security at December 31, 2021 were as follows (in thousands):
Amortized |
Unrealized |
Unrealized |
||||||||||
|
Cost |
|
Gains |
|
Losses |
|
Fair Value |
|||||
U.S. Treasury notes |
$ |
|
$ |
|
( |
$ |
|
|||||
Corporate debt securities |
|
— |
( |
|
||||||||
Total available-for-sale investments |
$ |
|
$ |
|
$ |
( |
$ |
|
||||
The following table summarizes the fair value of available-for-sale debt securities based on stated contractual maturities as of December 31, 2021 (in thousands):
|
Amortized Cost |
|
Fair Value |
|||
Due in less than one year |
$ |
|
$ |
|
||
Due between one and six years |
|
|
|
|
||
Total |
$ |
|
$ |
|
||
The amortized cost, gross unrealized gains, gross unrealized losses and fair value of available-for-sale debt securities by type of security at December 31, 2020 were as follows (in thousands):
Amortized |
Unrealized |
Unrealized |
||||||||||
|
Cost |
|
Gains |
|
Losses |
|
Fair Value |
|||||
U.S. Treasury notes |
$ |
|
|
$ |
|
|
$ |
( |
$ |
|
||
Total available-for-sale investments |
$ |
|
|
$ |
|
$ |
( |
$ |
|
|||
The primary objective of our investment portfolio is to enhance overall returns in an efficient manner while maintaining safety of principal, prudent levels of liquidity and acceptable levels of risk. Our investment policy limits interest-bearing security investments to certain types of debt and money market instruments issued by institutions with primarily investment-grade credit ratings, and it places restrictions on maturities and concentration by asset class and issuer.
F-25
We review our available-for-sale investments for other-than-temporary declines in fair value below our cost basis each quarter and whenever events or changes in circumstances indicate that the cost basis of an asset may not be recoverable. The evaluation is based on a number of factors, including the length of time and the extent to which the fair value has been below our cost basis, as well as adverse conditions related specifically to the security such as any changes to the credit rating of the security and the intent to sell or whether we will more likely than not be required to sell the security before recovery of its amortized cost basis. Our assessment of whether a security is other-than-temporarily impaired could change in the future based on new developments or changes in assumptions related to that particular security.
During the years ended December 31, 2021,2020 and 2019, we had $
Equity Investments
We held investments in equity securities with readily determinable fair values of $
Unrealized gains (losses) during 2021, 2020 and 2019 related to equity securities held at December 31, 2021, 2020 and 2019 are as follows (in thousands):
|
2021 |
|
2020 |
|
2019 |
||||
Net losses recognized during the year on equity securities |
$ |
( |
$ |
( |
$ |
( |
|||
Less: net losses recognized during the year on equity securities sold during the year |
|
— |
|
( |
— |
||||
Unrealized gains (losses) recognized during the year on equity securities still held at December 31, 2021, 2020 and 2019 |
$ |
( |
$ |
|
$ |
( |
|||
Note 5. Fair Value Measurements
We measure fair value based on the prices that would be received to sell an asset or paid to transfer a liability in an orderly transaction between market participants at the measurement date. Fair value measurements are based on a three-tier hierarchy that prioritizes the inputs used to measure fair value. These tiers include the following:
Level 1: Quoted prices (unadjusted) in active markets for identical assets or liabilities that are accessible at the measurement date. The fair value hierarchy gives the highest priority to Level 1 inputs.
Level 2: Observable prices that are based on inputs not quoted on active markets but corroborated by market data. These inputs include quoted prices for similar assets or liabilities; quoted market prices in markets that are not active; or other inputs that are observable or can be corroborated by observable market data for substantially the full term of the assets or liabilities.
Level 3: Unobservable inputs are used when little or no market data is available. The fair value hierarchy gives the lowest priority to Level 3 inputs.
In determining fair value, we utilize valuation techniques that maximize the use of observable inputs and minimize the use of unobservable inputs to the extent possible, as well as consider counterparty credit risk in the assessment of fair value.
We did not elect the fair value option, as allowed, to account for financial assets and liabilities that were not previously carried at fair value. Therefore, material financial assets and liabilities that are not carried at fair value, such as trade accounts receivable and payable, are reported at their historical carrying values.
F-26
The fair value of the contingent consideration liability for the
The carrying values of our assets that are required to be measured at fair value on a recurring basis as of December 31, 2021 and 2020 approximate fair value because of our ability to immediately convert these instruments into cash with minimal expected change in value which are classified in the table below in one of the three categories of the fair value hierarchy described above (in thousands):
|
Fair Value Measurements |
|||||||||||
|
Level 1 |
|
Level 2 |
|
Level 3 |
|
Total |
|||||
December 31, 2021 |
|
|
|
|
|
|
|
|
||||
Cash equivalents: |
|
|
|
|
|
|
|
|
||||
Money market mutual fund |
$ |
|
$ |
|
$ |
|
$ |
|
||||
Marketable equity securities: |
|
|
|
|
|
|
|
|
||||
Mutual funds |
|
|
|
|
|
|
|
|
||||
Available-for-sale debt securities: |
|
|
|
|
|
|
||||||
U.S. Treasury notes |
|
|
|
|
|
|
|
|
||||
Corporate debt securities |
|
|
|
|
||||||||
$ |
|
$ |
|
$ |
|
$ |
|
|||||
Fair Value Measurements |
||||||||||||
|
Level 1 |
|
Level 2 |
|
Level 3 |
|
Total |
|||||
December 31, 2020 |
||||||||||||
Cash equivalents: |
|
|
|
|
|
|
|
|
||||
Money market mutual fund |
$ |
|
$ |
|
$ |
|
$ |
|
||||
Marketable equity securities: |
|
|
|
|
|
|
|
|
||||
Mutual funds |
|
|
|
|
|
|
|
|
||||
Available-for-sale debt securities: |
|
|
|
|
|
|
|
|
||||
U.S. Treasury notes |
|
|
|
|
|
|
|
|
||||
$ |
|
$ |
|
$ |
|
$ |
|
|||||
Our equity securities and available-for-sale debt securities, including U.S. treasury notes are valued using inputs observable in active markets for identical securities and are therefore classified as Level 1 within the fair value hierarchy.
We did not have any financial liabilities measured at fair value on a recurring basis as of December 31, 2021.
We carry the Convertible Senior Notes (see Note 10) at face value less the unamortized discount and issuance costs on our consolidated balance sheets and present fair value for disclosure purposes only. We estimate the fair value of the Convertible Senior Notes using the net present value of the payments, discounted at an interest rate that is consistent with market and risk-adjusted interest rates, which is a Level 2 input.
The following table presents the estimated fair values and the carrying values (in thousands):
|
December 31, 2021 |
December 31, 2020 |
||||||||||
|
Carrying Value |
|
Fair Value |
|
Carrying Value |
|
Fair Value |
|||||
2026 Senior Notes |
$ |
|
$ |
|
$ |
— |
$ |
— |
||||
2025 Senior Notes |
$ |
|
$ |
|
$ |
|
$ |
|
||||
F-27
Note 6. Inventories
Inventories consist of the following (in thousands):
December 31, |
December 31, |
|||||
|
2021 |
|
2020 |
|||
Raw materials |
$ |
|
$ |
|
||
Work-in-process |
|
|
||||
Finished goods |
|
|
|
|
||
$ |
|
$ |
|
|||
The inventory balance at December 31, 2020 includes an $
Note 7. Property and Equipment
Property and equipment consist of the following (in thousands):
December 31, |
December 31, |
|||||
|
2021 |
|
2020 |
|||
Cryogenic shippers and data loggers |
$ |
|
$ |
|
||
Freezers |
|
|
||||
Furniture and fixtures |
|
|
|
|
||
Computers and software |
|
|
|
|
||
Machinery and equipment |
|
|
|
|
||
Trucks and autos |
|
|
||||
Leasehold improvements |
|
|
|
|
||
Buildings |
|
|
||||
Land |
|
|
||||
Fixed assets in process |
|
|
|
|
||
|
|
|
|
|||
Less accumulated depreciation and amortization |
|
( |
|
( |
||
$ |
|
$ |
|
|||
Total depreciation and amortization expense related to property and equipment amounted to $
The Company leases equipment under finance leases, with a total cost of $
F-28
Note 8. Goodwill and Intangible Assets
Goodwill
The following table represents the changes in the carrying value of goodwill for the years ended December 31, 2021 and 2020 (in thousands):
December 31, |
December 31, |
|||||
|
2021 |
|
2020 |
|||
Balance at beginning of year |
$ |
|
$ |
|
||
Foreign currency adjustment |
( |
— |
||||
Goodwill related to MVE acquisition |
|
|
|
|
||
Goodwill related to CRYOPDP acquisition |
|
( |
|
|
||
Goodwill related to CTSA and F-airGate acquisitions |
|
|
|
— |
||
Balance at end of year |
$ |
|
$ |
|
||
Intangible Assets
The following table presents our intangible assets as of December 31, 2021 (in thousands):
Weighted |
|||||||||||
Net |
Average |
||||||||||
Gross |
Accumulated |
Carrying |
Amortization |
||||||||
|
Amount |
|
Amortization |
|
Amount |
|
Period (years) |
||||
Non-compete agreement |
$ |
|
$ |
|
$ |
|
|
||||
Technology |
|
|
|
||||||||
Customer relationships |
|
|
|
||||||||
Trade name/trademark |
|
|
|
||||||||
Agent network |
|
|
|
||||||||
Order backlog |
|
|
— |
||||||||
Land use rights |
|
|
|
||||||||
Patents and trademarks |
|
|
|
||||||||
Total |
$ |
|
$ |
|
$ |
|
|||||
The following table presents our intangible assets as of December 31, 2020 (in thousands):
Weighted |
|||||||||||
Net |
Average |
||||||||||
Gross |
Accumulated |
Carrying |
Amortization |
||||||||
|
Amount |
|
Amortization |
|
Amount |
|
Period (years) |
||||
Non-compete agreement |
$ |
|
$ |
|
$ |
|
|
||||
Technology |
|
|
|
||||||||
Customer relationships |
|
|
|
||||||||
Trade name/trademark |
|
|
|
||||||||
Agent network |
|
|
|
||||||||
Order backlog |
|
|
— |
||||||||
Land use rights |
|
|
|
||||||||
Patents and trademarks |
|
|
|
|
|||||||
Total |
$ |
|
$ |
|
$ |
|
|||||
Amortization expense for intangible assets for the years ended December 31, 2021, 2020 and 2019 was $
F-29
Expected future amortization of intangible assets as of December 31, 2021 is as follows (in thousands):
Years Ending December 31, |
|
Amount |
|
2022 |
|
|
|
2023 |
|
|
|
2024 |
|
|
|
2025 |
|
|
|
2026 |
|
|
|
Thereafter |
|
|
|
$ |
|
||
Note 9. Accrued Compensation and Related Expenses
Accrued compensation and related expenses consist of the following (in thousands):
December 31, |
|
December 31, |
||||
|
2021 |
|
2020 |
|||
Accrued salaries and wages |
$ |
|
$ |
|
||
Accrued paid time off |
|
|
|
|
||
$ |
|
$ |
|
|||
Note 10. Convertible Senior Notes
Convertible Senior Notes payable consisted of the following at December 31, 2021 and 2020 (in thousands):
December 31, |
||||||
|
2021 |
|
2020 |
|||
Principal amount of 2025 Senior Notes |
$ |
|
$ |
|
||
Principal amount of 2026 Senior Notes |
|
|
|
— |
||
Less: unamortized debt issuance costs |
( |
( |
||||
Net carrying value of Convertible Senior Notes payable |
$ |
|
$ |
|
||
Interest expense incurred in connection with the Notes consisted of the following for the years ended December 31, 2021 and 2020 (in thousands):
December 31, |
|||||||||
|
2021 |
|
2020 |
|
2019 |
||||
Coupon interest |
$ |
|
$ |
|
$ |
— |
|||
Amortization of debt issuance costs |
|
|
|
— |
|||||
Total interest expense on Convertible Senior Notes |
$ |
|
$ |
|
$ |
— |
|||
As neither of the Convertible Senior Notes was outstanding in 2019, no interest expense related to these notes was recorded for the year ended December 31, 2019.
The Company’s 2025 Senior Notes and 2026 Senior Notes payable of $
2026 Senior Notes
On November 12, 2021, the Company issued $
F-30
underwriting discounts and commissions of $
Noteholders may convert their 2026 Senior Notes at their option into shares of the Company’s common stock in the following circumstances: (1) before the close of business on the business day immediately before September 1, 2026, noteholders have the right to convert their 2026 Senior Notes only upon the occurrence of certain events (e.g., if sale price per share of the Company’s common stock exceeds
The 2026 Senior Notes will be redeemable, in whole or in part (subject to certain limitations described below), at the Company’s option at any time, and from time to time, on or after December 6, 2024 and on or before the 41st scheduled trading day immediately before the maturity date, at a cash redemption price equal to the principal amount of the 2026 Senior Notes to be redeemed, plus accrued and unpaid interest, if any, to, but excluding, the redemption date, but only if certain liquidity conditions are satisfied and the last reported sale price per share of the Company’s common stock exceeds
F-31
The 2026 Senior Notes contain customary terms and events of default. If an event of default involving bankruptcy, insolvency, or reorganization events with respect to the Company (and not solely with respect to a significant subsidiary of the Company) occurs, then the principal amount of, and all accrued and unpaid interest on, the 2026 Senior Notes then outstanding will immediately become due and payable without any further action or notice by any person. If any other event of default (as defined in the Indenture) occurs and is continuing, then, the Trustee, by notice to the Company, or holders of at least
The 2026 Senior Notes are accounted for in accordance with ASC 470-20, Debt with Conversion and Other Options (“ASC 470-20”) and ASC 815-40, Contracts in Entity’s Own Equity (“ASC 815-40”). Under ASC 815-40, to qualify for equity classification (or nonbifurcation, if embedded) the instrument (or embedded feature) must be both (1) indexed to the issuer’s stock and (2) meet the requirements of the equity classification guidance. Based upon the Company’s analysis, it was determined the 2026 Senior Notes do contain embedded features indexed to its own stock, but do not meet the requirements for bifurcation and recognition as derivatives, and therefore do not need to be separately recognized. Accordingly, the proceeds received from the issuance of the 2026 Senior Notes were recorded as a single liability measured at amortized cost on the consolidated balance sheet.
The Company incurred approximately $
2025 Senior Notes
In May 2020, the Company issued $
F-32
At any time before the close of business on the scheduled trading day immediately before the maturity date, holders of the 2025 Senior Notes may convert their 2025 Senior Notes at their option into shares of the Company’s common stock. The 2025 Senior Notes were initially convertible into approximately
On or after June 5, 2023, we may redeem the 2025 Senior Notes at our option, in whole and not in part, at a cash redemption price equal to the principal amount of the 2025 Senior Notes to be redeemed, plus accrued and unpaid interest, if any, if:
| (1) |
The last reported sale price per share of the Company’s common stock exceeds |
| (2) | A registration statement covering the resale of the shares of the Company’s common stock issuable upon conversion of the Senior Notes is effective and available for use and is expected to remain effective and available during the redemption period as of the date the redemption notice is sent. |
The 2025 Senior Notes contain customary terms and events of default. If an event of default arising out of certain events of bankruptcy, insolvency, or reorganization involving the Company or a significant subsidiary (as set forth in the Indenture) occurs with respect to the Company, the principal amount of the 2025 Senior Notes and accrued and unpaid interest, if any, will automatically become immediately due and payable. If any other event of default (as defined in the Indenture) occurs and is continuing, either the Trustee or the holders of at least
The 2025 Senior Notes are accounted for in accordance with ASC 470-20, Debt with Conversion and Other Options (“ASC 470-20”) and ASC 815-40, Contracts in Entity’s Own Equity (“ASC 815-40”). Under ASC 815-40, to qualify for equity classification (or nonbifurcation, if embedded) the instrument (or embedded feature) must be both (1) indexed to the issuer’s stock and (2) meet the requirements of the equity classification guidance. Based upon the Company’s analysis, it was determined the 2025 Senior Notes do contain embedded features indexed to its own stock, but do not meet the requirements for bifurcation and recognition as derivatives, and therefore do not need to be separately recognized. Accordingly, the proceeds received from the issuance of the 2025 Senior Notes were recorded as a single liability measured at amortized cost on the consolidated balance sheet.
The Company incurred approximately $
On November 9, 2021, the Company entered into separate, privately negotiated note purchase agreements with a limited number of holders of its 2025 Senior Notes pursuant to which the Company repurchased approximately $
F-33
determined to have substantially different terms. The repurchase of the 2025 Senior Notes and issuance of the 2026 Senior Notes were deemed to have substantially different terms based on the present value of the cash flows. Therefore, the repurchase of the 2025 Senior Notes was accounted for as a debt extinguishment. The Company recorded $
In connection with the issuance of the 2025 Senior Notes, the Company entered into a registration rights agreement (the “Registration Rights Agreement”) to use its best efforts to file a registration statement for the resale of the 2025 Senior Notes and the shares of the Company’s common stock issuable upon conversion of the 2025 Senior Notes, to cause the registration statement to become effective by January 31, 2021, and to keep the registration statement continuously effective for a specified period of time. In December 2020, the Company filed an automatic shelf registration statement to register the resale of the 2025 Senior Notes and the shares of the Company’s common stock issuable upon conversion of the 2025 Senior Notes. If the Company fails to satisfy certain of its obligations under the Registration Rights Agreement (a “Registration Default”), it will be required to pay additional interest on the 2025 Senior Notes. Such additional interest will accrue at a rate per annum equal to
Note 11. Note Payable
In connection with the acquisition of CRYOPDP, the Company assumed an interest free unsecured note payable of €
Note 12. Leases
The Company has operating and finance leases for corporate offices and certain equipment. These leases have remaining lease terms of
The components of lease cost were as follows (in thousands):
December 31, |
|||||||||
|
2021 |
|
2020 |
|
2019 |
||||
Operating lease cost |
$ |
|
$ |
|
$ |
|
|||
Finance lease cost: |
|
||||||||
Amortization of right-of-use assets |
|
|
|
||||||
Interest on finance lease liabilities |
|
|
|
||||||
|
|
|
|
||||||
Total lease cost |
$ |
|
$ |
|
$ |
|
|||
F-34
Other information related to leases was as follows (in thousands):
Supplemental Cash Flows Information |
Year Ended December 31, |
|
||||||||
|
2021 |
|
2020 |
|
2019 |
|||||
Cash paid for amounts included in the measurement of lease liabilities: |
|
|
|
|||||||
Operating cash flows from operating leases |
|
$ |
|
|
$ |
|
|
$ |
|
|
Operating cash flows from finance leases |
|
$ |
|
|
$ |
|
|
$ |
|
|
Financing cash flows from finance leases |
$ |
|
$ |
|
$ |
|
||||
Right-of-use assets obtained in exchange for lease liabilities (in thousands): |
||||||||||
Operating leases |
|
$ |
|
|
$ |
|
|
$ |
|
|
Finance leases |
$ |
— |
$ |
|
$ |
— |
||||
December 31, |
|
||||
|
2021 |
|
2020 |
|
|
Weighted-Average Remaining Lease Term |
|
|
|||
Operating leases |
|
|
|||
Finance leases |
|
|
|||
Weighted-Average Discount Rate |
|
|
|
|
|
Operating leases |
|
% |
% |
||
Finance leases |
|
% |
% |
||
Future minimum lease payments under non-cancellable leases that have commenced as of December 31, 2021 were as follows (in thousands):
Years Ending December 31 |
|
Operating |
|
Finance |
||
2022 |
|
$ |
|
|
$ |
|
2023 |
|
|
|
|
||
2024 |
|
|
|
— |
||
2025 |
|
|
|
— |
||
2026 |
|
— |
||||
Thereafter |
|
|
|
— |
||
Total future minimum lease payments |
|
|
|
|
||
Less imputed interest |
|
( |
|
( |
||
Total |
$ |
|
$ |
|
||
Operating |
Finance |
|||||
Reported as of December 31, 2021 |
|
Leases |
|
Leases |
||
Current lease liabilities |
$ |
|
$ |
|
||
Noncurrent lease liabilities |
|
|
|
|
||
Total |
$ |
|
$ |
|
||
Note 13. Employee Benefit Plans
401(k) Plan
The Company provides a 401(k) Plan (“Plan”) to provide retirement and incidental benefits for our eligible U.S. based employees. Employees may contribute up to
F-35
Non-U.S. Employee Benefit Plans
Eligible employees outside the U.S. generally receive retirement benefits under various defined benefit plans and defined contribution plans based upon factors such as years of service and employee compensation levels. Eligibility is generally determined in accordance with local statutory requirements. The employee benefit plan costs and liabilities regarding the defined benefit plans are determined by actuarial valuations.
Employees of the Company who are in India participate in an employee benefit plan (the “Gratuity Plan”), which is required by local law and provides a lump sum payment to vested employees upon retirement, death, incapacitation, or termination of employment based on the respective employee’s salary and the tenure of employment. The benefit costs and liabilities regarding the Gratuity Plan are determined by actuarial valuations. The Company makes annual contributions to the employees’ gratuity fund established with Life Insurance Corporation of India, which calculates the annual contribution required to be made by the Company and manages the Gratuity Plan, including any required payouts. The Gratuity Plan is partially funded. The obligation under the Gratuity Plan is not significant at December 31, 2021.
Benefit costs associated with the non-U.S. employee benefit plans totaled $
Note 14. Commitments and Contingencies
Facility and Equipment Leases
We lease various corporate, global logistics and supply chain centers, biostorage, manufacturing, and research and development facilities in Tennessee, California, New Jersey, the Netherlands, Texas, Georgia, Minnesota, China, Portugal and France under operating leases. These lease agreements contain certain scheduled annual rent increases which are accounted for on a straight-line basis. In addition, we lease certain equipment which expires through November 2024 (See Note 12).
Employment Agreements
We have entered into employment agreements with certain of our officers under which payment and benefits would become payable in the event of termination by us for any reason other than cause, or upon a change in control of our Company, or by the employee for good reason.
Litigation
The Company may become a party to product litigation in the normal course of business. The Company accrues for open claims based on its historical experience and available insurance coverage. We record a loss contingency when it is probable that a liability has been incurred and the amount of the loss can be reasonably estimated. We also disclose material contingencies when we believe a loss is not probable but reasonably possible. Accounting for contingencies requires us to use judgment related to both the likelihood of a loss and the estimate of the amount or range of loss. The outcomes of our legal proceedings are inherently unpredictable, subject to significant uncertainties, and could be material to our financial condition, results of operations, and cash flows for a particular period.
Indemnities and Guarantees
The Company has made certain indemnities and guarantees, under which it may be required to make payments to a guaranteed or indemnified party, in relation to certain actions or transactions. The guarantees and indemnities do not provide for any limitation of the maximum potential future payments the Company could be obligated to make. Historically, the Company has not been obligated nor incurred any payments for these obligations and, therefore, no liabilities have been recorded for these indemnities and guarantees in the accompanying consolidated balance sheets.
The Company indemnifies its directors, officers, employees and agents, as permitted under the laws of the States of California and Nevada. In connection with its facility and equipment leases, the Company has indemnified its lessors for certain claims arising from the use of the facilities and equipment. The duration of the guarantees and indemnities varies and is generally tied to the life of the agreements.
F-36
Note 15. Stockholders’ Equity
Authorized Stock
The Company has
Common Stock Issuances For Services
During the year ended December 31, 2021,
During the year ended December 31, 2020,
During the year ended December 31, 2019,
November 2021 Registered Direct Placement and Stock Purchase Agreements
Concurrent with the issuance of the 2026 Senior Notes in November 2021, the Company conducted a registered direct placement of
January 2021 Public Offering
On January 25, 2021, the Company completed an underwritten public offering of
Blackstone Private Placement
In connection with the MVE Acquisition, on October 1, 2020 (the “Closing Date”), the Company completed a private placement with an investment vehicle of funds affiliated with The Blackstone Group Inc. (collectively, “Blackstone”), consisting of (i)
F-37
The Series C Preferred Stock ranks senior to the shares of the Company’s common stock, with respect to dividend rights and rights upon the voluntary or involuntary liquidation, dissolution, or winding up of the affairs of the Company (a “Liquidation”). The Series C Preferred Stock has the following rights, preferences and privileges:
Dividend Rights. Holders of the Series C Preferred Stock (the “Holders”) are entitled to dividends at the rate of
Liquidation Preference. Upon a Liquidation, each share of Series C Preferred Stock is entitled to receive an amount per share equal to the greater of (i) $
Conversion Features. The Series C Preferred Stock is convertible at the option of the Holders at any time into shares of common stock at a conversion price of $
After the second anniversary of the Closing Date, subject to certain conditions, the Company may at its option require conversion of all of the outstanding shares of the Series C Preferred Stock to common stock if, for at least
On the October 1, 2020 issuance date, the effective conversion price per share was less than the fair value of the underlying common stock and, as a result, the Company determined that there was a beneficial conversion feature on that date. Accordingly, the Company recognized the resulting beneficial conversion feature amount of $
Additionally, the Company determined that the nature of the Series C Preferred Stock is more akin to an equity instrument and that the economic characteristics and risks of the embedded conversion options are clearly and closely related to the Series C Preferred Stock. As such, the conversion options were not required to be bifurcated from the host under ASC 815, Derivatives and Hedging.
Since the paid-in-kind dividends are nondiscretionary, the Company measures the beneficial conversion feature of the paid-in-kind dividends on the issuance date of the preferred stock and records such amount when the paid-in-kind dividends are accrued. Accordingly, the associated paid-in-kind dividends for the year ended December 31, 2020 generated a beneficial conversion feature amount of $
Redemption Rights. The Company may redeem the Series C Preferred Stock for cash, as follows:
| (1) |
At any time beginning five years after the Closing Date (but prior to six years after the Closing Date), all of the Series C Preferred Stock at a price equal to |
| (ii) |
At any time beginning six years after the Closing Date, all of the Series C Preferred Stock at a price equal to |
Upon a “Fundamental Change” (involving a change of control or de-listing of the Company as further described in the Certificate of Designation), each Holder has the right to require the Company to redeem all or any part of the Holder’s Series C Preferred
F-38
Stock for an amount equal to the Liquidation Preference plus any accrued and unpaid dividends. If the Company does not have sufficient funds legally available to pay the repurchase price, then the Company is required to (a) pay the maximum amount of the repurchase price that can be paid out of funds legally available for payment, and (b) purchase any shares of the Series C Preferred Stock not purchased because of the foregoing limitations at the repurchase price as soon as practicable after the Company is able to make such purchase out of assets legally available for the purchase of such shares. If the Company fails to pay the repurchase price in full when due, then the Company will pay dividends on such shares not repurchased at a rate of
The Company evaluated the Series C Preferred Stock for liability or equity classification under the applicable accounting guidance including ASC 480, Distinguishing Liabilities from Equity, and determined that equity treatment was appropriate because the Series C Preferred Stock did not meet the definition of the liability instruments defined thereunder for convertible instruments. Specifically, the convertible preferred shares are not mandatorily redeemable and do not embody an obligation to buy back the shares outside of the Company’s control in a manner that could require the transfer of assets. Additionally, the Company determined that the Series C Preferred Stock would be recorded as permanent equity given that they are not redeemable for cash or other assets (i) on a fixed or determinable date, (ii) at the option of the holder, or (iii) upon the occurrence of an event that is not solely within control of the Company.
The Company also evaluated the embedded put and call options within the Series C Preferred Stock in accordance with the accounting guidance for derivatives to determine if bifurcation is required. The Company determined that the economic characteristics and risks of the embedded put and call options are not clearly and closely related to the Series C Preferred Stock. Therefore, the Company assessed the put and call options further and determined they did not meet the definition of a derivative under ASC 815.
Under the same analysis, the Company determined that the economic characteristics and risks of the embedded participating dividend feature are considered clearly and closely related to the equity host. Accordingly, the participating dividend feature is not required to be bifurcated under ASC 815. Also, the Company determined that the value of the contingent dividend feature is minimal and insignificant relative to the other components of the Series C Preferred Stock due to the circumstances surrounding the scenarios under which the provision would be triggered.
Voting Rights. Holders of the Series C Preferred Stock are generally entitled to vote with the holders of the shares of common stock on an as-converted basis, subject to certain Nasdaq voting limitations, if applicable. Also, the consent of the Holders of a majority of the outstanding shares of the Series C Preferred Stock is required with respect to (i) amendments to the Company’s organizational documents that have an adverse effect on the Holders of the Series C Preferred Stock, and (ii) issuances by the Company of securities that are senior to, or equal in priority with, the Series C Preferred Stock. Holders of the Series C Preferred Stock have the right to nominate for election
Registration Rights. Holders of the Series C Preferred Stock have certain customary registration rights with respect to the Series C Preferred Stock and the shares of common stock into which they are converted, pursuant to the terms of a registration rights agreement. The Company is required to file within 90 days of the Closing Date, and use its commercially reasonable efforts to cause to go effective as promptly as practicable, a registration statement covering the sale or distribution of common stock issued or issuable upon conversion of the Series C Preferred Stock. In December 2020, the Company filed an automatic shelf registration statement to register the resale of the common stock issued or issuable upon conversion of the Series C Preferred Stock.
Blackstone Conversion
On February 5, 2021, the Company received a waiver and conversion notice from Blackstone Freeze Parent L.P. and Blackstone Tactical Opportunities Fund – FD L.P. to convert an aggregate of
F-39
Share Repurchase Program
In October 2019, the Company’s Board of Directors authorized a share repurchase program (the “Repurchase Program”) authorizing repurchase of common stock in the amount of up to $
June 2019 Public Offering
On June 24, 2019, the Company completed an underwritten public offering of
Common Stock Reserved for Future Issuance
As of December 31, 2021, approximately $
Exercise of stock options |
|
|
Vesting of restricted stock units |
|
|
Conversion of Series C Preferred stock |
|
|
Conversion of convertible 2026 Senior Notes |
|
|
Conversion of convertible 2025 Senior Notes |
|
|
Total shares of common stock reserved for future issuances |
|
|
Note 16. Stock-Based Compensation
Warrants
We typically issue warrants to purchase shares of our common stock to investors as part of a financing transaction or in connection with services rendered by placement agents and consultants. A summary of warrant activity is as follows:
|
|
|
Weighted- |
|
||||||
Weighted- |
Average |
|||||||||
Average |
Remaining |
Aggregate |
||||||||
Number of |
Exercise |
Contractual |
Intrinsic |
|||||||
|
Shares |
|
Price/Share |
|
Term (Years) |
|
Value (1) |
|||
Outstanding — December 31, 2018 |
|
|
$ |
|
|
|
|
|
||
Issued |
|
— |
|
— |
|
|
|
|
||
Exercised |
|
( |
|
|
|
|
|
|
||
Expired |
|
( |
|
|
|
|
|
|
||
Outstanding — December 31, 2019 |
|
|
|
|
|
|
|
|||
Issued |
|
— |
|
— |
|
|
|
|
||
Exercised |
|
( |
|
|
|
|
|
|
||
Expired |
|
( |
|
|
|
|
|
|
||
Outstanding — December 31, 2020 |
|
— |
$ |
— |
|
$ |
— |
|||
Vested (exercisable) — December 31, 2020 |
|
— |
$ |
— |
|
$ |
— |
|||
F-40
During the year ended December 31, 2020, the Company issued
During the year ended December 31, 2019, the Company issued
The total intrinsic value of warrants exercised during the years ended December 31, 2021, 2020 and 2019 was $
Stock Options
We have five stock incentive plans: the 2002 Stock Incentive Plan (the “2002 Plan”), the 2009 Stock Incentive Plan (the “2009 Plan”), the 2011 Stock Incentive Plan (the “2011 Plan”), the 2015 Omnibus Equity Incentive Plan (the “2015 Plan”), and the 2018 Omnibus Equity Incentive Plan (the “2018 Plan”) (collectively, the “Plans”). The 2002 Plan, the 2009 Plan, the 2011 Plan and the 2015 Plan (the “Prior Plans”) have been superseded by the 2018 Plan. In May 2018, the stockholders approved the 2018 Plan for issuances up to an aggregate of
During the years ended December 31, 2021, 2020 and 2019, we granted stock options at exercise prices equal to or greater than the quoted market price of our common stock on the grant date. The fair value of each option grant was estimated on the date of grant using Black-Scholes with the following assumptions:
December 31, |
|||||||
|
2021 |
|
2020 |
|
2019 |
||
Expected life (years) |
- |
- |
- |
||||
Risk-free interest rate |
|
% |
% |
% |
|||
Volatility |
|
% |
% |
% |
|||
Dividend yield |
|
||||||
The expected option life assumption is estimated based on the simplified method. Accordingly, the Company has utilized the average of the contractual term of the options and the weighted average vesting period for all options to calculate the expected option term. The risk-free interest rate assumption is based upon observed interest rates appropriate for the expected term of our employee stock options. The expected volatility is based on the average of the historical volatility and the implied volatility of our stock commensurate with the expected life of the stock-based award. We do not anticipate paying dividends on the common stock in the foreseeable future.
We recognize stock-based compensation cost on a straight-line basis over the vesting period. Stock-based compensation expense is recognized only for those awards that ultimately vest.
Total stock-based compensation expense related to our share-based payment awards is comprised of the following (in thousands):
Year Ended December 31, |
|||||||||
|
2021 |
|
2020 |
|
2019 |
||||
Cost of revenues |
$ |
|
$ |
|
$ |
|
|||
Selling, general and administrative |
|
|
|
|
|
|
|||
Engineering and development |
|
|
|
|
|
|
|||
$ |
|
$ |
|
$ |
|
||||
For the year ended December 31, 2019, we recognized expense of $
F-41
grants. Of this amount, $
A summary of stock option activity is as follows:
Weighted- |
||||||||||
Weighted- |
Average |
|||||||||
Average |
Remaining |
Aggregate |
||||||||
Number of |
Exercise |
Contractual |
Intrinsic |
|||||||
|
Shares |
|
Price/Share |
|
Term (Years) |
|
Value (1) |
|||
Outstanding — December 31, 2019 |
|
|
$ |
|
|
|
|
|
||
Granted (weighted-average fair value of $ |
|
|
|
|
|
|
|
|||
Exercised |
|
( |
|
|
|
|
|
|
||
Forfeited |
|
( |
|
|
|
|
|
|
||
Expired |
|
( |
|
|
|
|
|
|
||
Outstanding — December 31, 2020 |
|
|
|
|
|
|
|
|||
Granted (weighted-average fair value of $ |
|
|
|
|||||||
Exercised |
|
( |
|
|
|
|
|
|||
Forfeited |
|
( |
|
|
|
|
|
|||
Outstanding — December 31, 2021 |
|
|
$ |
|
|
$ |
|
|||
Vested (exercisable) — December 31, 2021 |
|
|
$ |
|
|
$ |
|
|||
Expected to vest after December 31, 2021 (unexercisable) |
|
|
$ |
|
|
$ |
|
|||
| (1) |
Aggregate intrinsic value represents the difference between the exercise price of the option and the closing market price of the common stock on December 31, 2021, which was $ |
The following table summarizes information with respect to stock options outstanding and exercisable at December 31, 2021:
Weighted- |
||||||||||||
Average |
Weighted- |
Weighted- |
||||||||||
Remaining |
Average |
Average |
||||||||||
Number |
Contractual |
Exercise |
Number |
Exercise |
||||||||
Exercise Price |
|
Outstanding |
|
Life (Years) |
|
Price |
|
Exercisable |
|
Price |
||
$1.87 – 3.07 |
|
|
|
$ |
|
|
|
$ |
|
|||
$3.19 – 3.44 |
|
|
|
$ |
|
|
|
$ |
|
|||
$4.80 – 4.92 |
|
|
|
$ |
|
|
|
$ |
|
|||
$5.00 – 7.67 |
|
|
|
$ |
|
|
|
$ |
|
|||
$7.80 – 8.65 |
|
|
|
$ |
|
|
|
$ |
|
|||
$9.29 – 12.79 |
|
|
|
$ |
|
|
|
$ |
|
|||
$13.37 – 16.95 |
|
|
|
$ |
|
|
|
$ |
|
|||
$17.36 – 73.15 |
|
|
|
$ |
|
|
|
$ |
|
|||
|
|
|
|
|||||||||
As of December 31, 2021, there was unrecognized compensation expense of $
The total intrinsic value of options exercised during the years ended December 31, 2021, 2020 and 2019 was $
F-42
Restricted stock units
A summary of our restricted stock unit activity is as follows:
|
|
Weighted Average |
|||
Number of Restricted |
Fair Value per |
||||
Stock Units |
Share |
||||
Outstanding – December 31, 2020 |
|
— |
$ |
— |
|
Granted |
|
|
|
|
|
Forfeited |
|
( |
|
|
|
Share issuance |
|
— |
|
— |
|
Outstanding – December 31, 2021 |
|
|
$ |
|
|
For the year ended December 31, 2021, we recorded stock-based compensation expense on our issued restricted stock units of $
Note 17. Income Taxes
Loss before provision for income taxes was attributed to the following jurisdictions for the years ended December 31, 2021, 2020 and 2019 (in thousands):
Years Ended December 31, |
|||||||||
|
2021 |
|
2020 |
|
2019 |
||||
United States |
$ |
( |
$ |
( |
$ |
( |
|||
Foreign |
|
( |
|
|
|
|
|||
$ |
( |
$ |
( |
$ |
( |
||||
The provision for income taxes consists of the following for the years ended December 31, 2021, 2020 and 2019 (in thousands):
Years Ended December 31, |
|||||||||
|
2021 |
|
2020 |
|
2019 |
||||
Current: |
|
|
|
|
|
|
|||
Federal |
$ |
|
$ |
|
$ |
— |
|||
State |
|
|
|
|
|
|
|||
Foreign |
|
|
|
|
|
— |
|||
Total current expense |
|
|
|
|
|
|
|||
Deferred: |
|
|
|
||||||
Federal |
|
( |
|
( |
|
( |
|||
State |
|
( |
|
( |
|
|
|||
Foreign |
|
( |
|
( |
|
|
|||
Change in valuation allowance |
|
|
|
|
|
|
|||
Total deferred expense |
|
( |
|
( |
|
|
|||
Total provision for (benefit from) income taxes |
$ |
|
$ |
( |
$ |
|
|||
F-43
Significant components of the Company's deferred tax assets and liabilities as of December 31, 2021 and 2020 are shown below (in thousands):
December 31, |
||||||
|
2021 |
|
2020 |
|||
Deferred tax assets: |
|
|||||
Net operating loss carryforward |
$ |
|
$ |
|
||
Expenses recognized for granting of options and warrants |
|
|
|
|
||
Accrued expenses and reserves |
|
|
|
|
||
Lease liability |
|
|
||||
Total deferred tax assets |
|
|
||||
Valuation allowance |
|
( |
|
( |
||
$ |
|
$ |
|
|||
Deferred tax liabilities: |
||||||
Goodwill |
$ |
( |
$ |
( |
||
Right-of-use assets |
( |
( |
||||
Intangibles |
( |
( |
||||
Unremitted foreign earnings |
( |
— |
||||
Total deferred tax liability |
( |
( |
||||
Net deferred tax liability |
$ |
( |
$ |
( |
||
Our net deferred tax liability as presented in our consolidated balance sheet consists of the following items (in thousands):
December 31, |
||||||
|
2021 |
|
2020 |
|||
Deferred tax assets |
$ |
|
$ |
|
||
Deferred tax liabilities |
|
( |
|
( |
||
Net deferred tax liability |
$ |
( |
$ |
( |
||
The Company has recorded a net deferred tax liability in jurisdictions where taxable temporary differences from indefinite-lived intangible assets do not support the realization of deferred tax assets which have finite carryover periods. In addition, the Company has recorded a net deferred tax liability in jurisdictions where taxable temporary differences exceed deductible temporary.
The provision for (benefit from) income taxes differs from that computed using the federal statutory rate applied to loss before provision for income taxes as follows (in thousands):
December 31, |
|||||||||
|
2021 |
|
2020 |
|
2019 |
||||
Computed tax benefit at federal statutory rate |
$ |
( |
$ |
( |
$ |
( |
|||
State tax, net of federal benefit |
|
( |
|
( |
|
( |
|||
Non-deductible loss on debt extinguishment |
|
— |
— |
||||||
Stock compensation |
|
( |
|
( |
|
|
|||
Deemed foreign dividend income |
|
— |
— |
||||||
Interest expense |
— |
— |
|
||||||
Permanent differences and other |
|
|
|
( |
|
( |
|||
Transaction cost |
— |
|
— |
||||||
Executive compensation |
|
|
|
||||||
Rate changes |
|
|
— |
||||||
Contingencies |
|
|
|
|
|
|
|||
Valuation allowance |
|
|
|
|
|
|
|||
$ |
|
$ |
( |
$ |
|
||||
F-44
At December 31, 2021, the Company has federal and state net operating loss carryforwards of approximately $
Utilization of the net operating loss (“NOL”) and research and development (“R&D”) carryforwards might be subject to a substantial annual limitation due to ownership change limitations that may have occurred or that could occur in the future, as required by Section 382 of the Internal Revenue Code of 1986, as amended (the “Code”), as well as similar state and foreign provisions. These ownership changes may limit the amount of NOL and R&D credit carryforwards that can be utilized annually to offset future taxable income and tax, respectively. In general, an “ownership change” as defined by Section 382 of the Code results from a transaction or series of transactions over a three-year period resulting in an ownership change of more than 50 percentage points of the outstanding stock of a company by certain stockholders or public groups. Since the Company’s formation, the Company has raised capital through the issuance of capital stock on several occasions which, combined with the purchasing stockholders’ subsequent disposition of those shares, may have resulted in such an ownership change, or could result in an ownership change in the future upon subsequent capital stock transactions.
The Company has not completed a study to assess whether an ownership change or changes has occurred. If the Company has experienced an ownership change, utilization of the NOL or R&D credit carryforwards would be subject to an annual limitation under Section 382 of the Code, which is determined by first multiplying the value of the Company’s stock at the time of the ownership change by the applicable long-term tax-exempt rate. Any limitation may result in expiration of a portion of the NOL or R&D credit carryforwards before utilization. Further, until a study is completed and any limitation is known, no amounts are being considered as an uncertain tax position or disclosed as an unrecognized tax benefit. Due to the existence of the valuation allowance, future changes in the Company’s unrecognized tax benefits will not impact its effective tax rate. Any carryforwards that will expire prior to utilization as a result of such limitations will be removed from deferred tax assets with a corresponding reduction of the valuation allowance.
The 2017 tax reform act amended the Internal Revenue Code (“Code”), effective for amounts paid or incurred in tax years beginning after December 31, 2021, to eliminate the immediate expensing of research and experimental expenditures (“R&E”) and to require taxpayers to charge their R&E expenditures and software development costs (collectively, R&E expenditures) to a capital account. Capitalized costs are required to be amortized over five years (15 years for expenditures attributable to foreign research). Additionally, the R&E credit may only be claimed for costs that are eligible to be treated as R&E expenditures under the Code. It is expected that any amounts treated as qualified research expenditures for purposes of the R&E credit also will be capitalized under Code.
A reconciliation of the beginning and ending amounts of unrecognized tax positions are as follows (in thousands):
December 31, |
|||||||||
|
2021 |
|
2020 |
|
2019 |
||||
Unrecognized tax positions, beginning of period |
$ |
|
$ |
|
$ |
|
|||
Gross increase – current period tax positions |
|
|
|
|
|
|
|||
Gross decrease – prior period tax positions |
|
— |
|
( |
|
— |
|||
Gross increase – prior period tax positions |
|
|
|
|
|
|
|||
Expiration of statute of limitations |
|
— |
|
— |
|
— |
|||
Unrecognized tax positions, end of period |
$ |
|
$ |
|
$ |
|
|||
If recognized, none of the unrecognized tax positions would impact the Company’s income tax benefit or effective tax rate as long as the Company’s deferred tax assets remain subject to a full valuation allowance. The Company does not expect any significant increases or decreases to the Company's unrecognized tax positions within the next 12 months.
We recognize interest accrued related to unrecognized tax benefits (“UTBs”) and penalties as income tax expense. We accrued an immaterial amount of interest expense during 2021 in our statement of operations, and as of December 31, 2021, have an immaterial accrual for interest in our consolidated balance sheet.
F-45
Due to the NOL carryforwards, the U.S. federal and state returns remain open to examination by the Internal Revenue Service and state taxing jurisdictions for all years beginning with the year ended March 31, 2002. Our foreign subsidiaries are generally subject to examination three years following the year on which the tax obligation originated. The years subject to audit may be extended if the entity substantially understates corporate income tax. The Company’s subsidiary in India is currently under examination by the Office of the Commissioner of Income Tax in India for the 2012-2013, 2013-2014 and 2015-2016 tax periods. Other than India, the Company does not have any foreign subsidiaries currently under audit by their local tax authorities.
Note 18. Quarterly Financial Data (Unaudited)
A summary of quarterly financial data is as follows (in thousands):
Quarter Ended |
||||||||||||
|
March 31 |
|
June 30 |
|
September 30 |
|
December 31 |
|||||
Year ended December 31, 2021 |
||||||||||||
Total revenues |
$ |
|
$ |
|
$ |
|
$ |
|
||||
Gross margin |
$ |
|
$ |
|
$ |
|
$ |
|
||||
Loss from operations |
$ |
( |
$ |
( |
$ |
( |
$ |
( |
||||
Net loss attributable to common stockholders |
$ |
( |
$ |
( |
$ |
( |
$ |
( |
||||
Net loss per share attributable to common stockholders - basic and diluted |
$ |
( |
$ |
( |
$ |
( |
$ |
( |
||||
Year ended December 31, 2020 |
|
|
|
|
|
|
|
|
||||
Total revenues |
$ |
|
$ |
|
$ |
|
$ |
|
||||
Gross margin |
$ |
|
$ |
|
$ |
|
$ |
|
||||
Loss from operations |
$ |
( |
$ |
( |
$ |
( |
$ |
( |
||||
Net loss attributable to common stockholders |
$ |
( |
$ |
( |
$ |
( |
$ |
( |
||||
Net loss per share attributable to common stockholders - basic and diluted |
$ |
( |
$ |
( |
$ |
( |
$ |
( |
||||
Year ended December 31, 2019 |
|
|
|
|
|
|
|
|
||||
Total revenues |
$ |
|
$ |
|
$ |
|
$ |
|||||
Gross margin |
$ |
|
$ |
|
$ |
|
$ |
|||||
Loss from operations |
$ |
( |
$ |
( |
$ |
( |
$ |
( |
||||
Net loss |
$ |
( |
$ |
( |
$ |
( |
$ |
( |
||||
Net loss per share - basic and diluted |
$ |
( |
$ |
( |
$ |
( |
$ |
( |
||||
Earnings per basic and diluted shares are computed independently for each of the quarters presented based on basic and diluted shares outstanding per quarter and, therefore, may not sum to the totals for the periods shown.
In the fourth quarter of 2019, management identified an error in the Company's historical interim financial statements for the third quarter of fiscal year 2019 relating to its stock-based compensation expense. Specifically, in the third quarter of 2019, the Company incorrectly recorded $
Note 19. Subsequent Events
On January 25, 2022, a fire occurred at the MVE Biological Solutions manufacturing facility located in New Prague, Minnesota. The New Prague facility manufactures aluminum dewars and is one of MVE Biological Solutions’ three global manufacturing facilities. There were no injuries reported and damage was limited to a portion of the facility. As a consequence of the fire damage, the New Prague manufacturing operations were curtailed on an interim basis until the necessary repairs were completed. Production was resumed at the New Prague facility during the week of February 14, 2022 and is expected to ramp up to full production during the first quarter of 2022. The Company anticipates the revenue impact of $
F-46